rBMSC/Cav-1F92A Mediates Oxidative Stress in PAH Rat by Regulating SelW/14-3-3 η and CA1/Kininogen Signal Transduction
- PMID: 31781243
- PMCID: PMC6855026
- DOI: 10.1155/2019/6768571
rBMSC/Cav-1F92A Mediates Oxidative Stress in PAH Rat by Regulating SelW/14-3-3 η and CA1/Kininogen Signal Transduction
Abstract
Background/objectives: Carbonic anhydrase 1 (CA1)/kininogen and selenoprotein W (SelW)/14-3-3η signal transduction orchestrate oxidative stress, which can also be regulated by nitric oxide (NO). The mutated caveolin-1 (Cav-1F92A) gene may enhance NO production. This study explored the effect of Cav-1F92A-modified rat bone marrow mesenchymal stem cells (rBMSC/Cav-1F92A) on oxidative stress regulation through CA1/kininogen and SelW/14-3-3η signal transduction in a rat model of monocrotaline- (MCT-) induced pulmonary arterial hypertension (PAH).
Method: PAH was induced in rats through the subcutaneous injection of MCT. Next, rBMSC/Vector (negative control), rBMSC/Cav-1, rBMSC/Cav-1F92A, or rBMSC/Cav-1F92A+L-NAME were administered to the rats. Changes in pulmonary hemodynamic and vascular morphometry and oxidative stress levels were evaluated. CA1/kininogen and SelW/14-3-3η signal transduction, endothelial nitric oxide synthase (eNOS) dimerization, and eNOS/NO/sGC/cGMP pathway changes were determined through real-time polymerase chain reaction, Western blot, or immunohistochemical analyses.
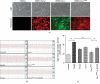
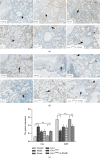
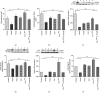
Results: In MCT-induced PAH rats, rBMSC/Cav-1F92A treatment reduced right ventricular systolic pressure, vascular stenosis, and oxidative stress; downregulated CA1/kininogen signal transduction; upregulated SelW/14-3-3η signal transduction; and reactivated the NO pathway.
Conclusions: In a rat model of MCT-induced PAH, rBMSC/Cav-1F92A reduced oxidative stress by regulating CA1/kininogen and SelW/14-3-3η signal transduction.
Copyright © 2019 Wan-cheng Yu et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Gene expression profiling of common signal transduction pathways affected by rBMSCs/F92A-Cav1 in the lungs of rat with pulmonary arterial hypertension.Biomed Pharmacother. 2016 Oct;83:100-106. doi: 10.1016/j.biopha.2016.06.028. Epub 2016 Jun 23. Biomed Pharmacother. 2016. PMID: 27470556
-
Mesenchymal Stem Cells Expressing eNOS and a Cav1 Mutant Inhibit Vascular Smooth Muscle Cell Proliferation in a Rat Model of Pulmonary Hypertension.Heart Lung Circ. 2017 May;26(5):509-518. doi: 10.1016/j.hlc.2016.08.002. Epub 2016 Sep 9. Heart Lung Circ. 2017. PMID: 27771236
-
Dissecting Molecular Mechanisms Underlying Pulmonary Vascular Smooth Muscle Cell Dedifferentiation in Pulmonary Hypertension: Role of Mutated Caveolin-1 (Cav1F92A)-Bone Marrow Mesenchymal Stem Cells.Heart Lung Circ. 2019 Oct;28(10):1587-1597. doi: 10.1016/j.hlc.2018.08.002. Epub 2018 Sep 7. Heart Lung Circ. 2019. PMID: 30262154
-
Caveolin-1 scaffolding domain residue phenylalanine 92 modulates Akt signaling.Eur J Pharmacol. 2015 Nov 5;766:46-55. doi: 10.1016/j.ejphar.2015.09.033. Epub 2015 Sep 25. Eur J Pharmacol. 2015. PMID: 26409042
-
Molecular control of nitric oxide synthesis through eNOS and caveolin-1 interaction regulates osteogenic differentiation of adipose-derived stem cells by modulation of Wnt/β-catenin signaling.Stem Cell Res Ther. 2016 Dec 7;7(1):182. doi: 10.1186/s13287-016-0442-9. Stem Cell Res Ther. 2016. PMID: 27927230 Free PMC article.
Cited by
-
Phoenixin 20 ameliorates pulmonary arterial hypertension via inhibiting inflammation and oxidative stress.Aging (Albany NY). 2024 Mar 19;16(6):5027-5037. doi: 10.18632/aging.205468. Epub 2024 Mar 19. Aging (Albany NY). 2024. PMID: 38517365 Free PMC article.
-
The critical roles of caveolin-1 in lung diseases.Front Pharmacol. 2024 Sep 24;15:1417834. doi: 10.3389/fphar.2024.1417834. eCollection 2024. Front Pharmacol. 2024. PMID: 39380904 Free PMC article. Review.
-
Dissecting molecular mechanisms underlying ferroptosis in human umbilical cord mesenchymal stem cells: Role of cystathionine γ-lyase/hydrogen sulfide pathway.World J Stem Cells. 2023 Nov 26;15(11):1017-1034. doi: 10.4252/wjsc.v15.i11.1017. World J Stem Cells. 2023. PMID: 38058959 Free PMC article.
-
Mechanistic Insight into the Enhanced Anti-Pulmonary Hypertension Efficacy of Wogonin Co-Amorphous.Pharmaceutics. 2025 May 30;17(6):724. doi: 10.3390/pharmaceutics17060724. Pharmaceutics. 2025. PMID: 40574037 Free PMC article.
-
Oxidative Stress and Antioxidative Therapy in Pulmonary Arterial Hypertension.Molecules. 2022 Jun 9;27(12):3724. doi: 10.3390/molecules27123724. Molecules. 2022. PMID: 35744848 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

