Ethyl Vanillin Protects against Kidney Injury in Diabetic Nephropathy by Inhibiting Oxidative Stress and Apoptosis
- PMID: 31781325
- PMCID: PMC6875338
- DOI: 10.1155/2019/2129350
Ethyl Vanillin Protects against Kidney Injury in Diabetic Nephropathy by Inhibiting Oxidative Stress and Apoptosis
Abstract
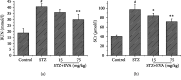
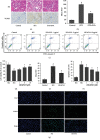
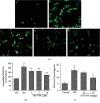
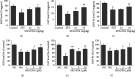
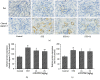
Diabetes-induced oxidative stress and apoptosis is regarded as a critical role in the pathogenesis of diabetic nephropathy (DN). Treating diabetes-induced kidney damage and renal dysfunction has been thought a promising therapeutic option to attenuate the development and progression of DN. In this study, we investigated the renoprotective effect of ethyl vanillin (EVA), an active analogue of vanillin isolated from vanilla beans, on streptozotocin- (STZ-) induced rat renal injury model and high glucose-induced NRK-52E cell model. The EVA treatment could strongly improve the deterioration of renal function and kidney cell apoptosis in vivo and in vitro. Moreover, treating with EVA significantly decreased the level of MDA and reactive oxygen species (ROS) and stabilized antioxidant enzyme system in response to oxidative stress by enhancing the activity of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) in vivo and in vitro. Furthermore, EVA also markedly suppressed cleaved caspase-3, Bax, and nuclear transcription factor erythroid 2-related factor (Nrf2) expression in STZ-induced rats. Therefore, these results of our investigation provided that EVA might protect against kidney injury in DN by inhibiting oxidative stress and cell apoptosis.
Copyright © 2019 Yuna Tong et al.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Antioxidant and Antiapoptotic effect of aqueous extract of Pueraria tuberosa (Roxb. Ex Willd.) DC. On streptozotocin-induced diabetic nephropathy in rats.BMC Complement Altern Med. 2018 May 11;18(1):156. doi: 10.1186/s12906-018-2221-x. BMC Complement Altern Med. 2018. PMID: 29751837 Free PMC article.
-
Diabetes aggravates renal ischemia and reperfusion injury in rats by exacerbating oxidative stress, inflammation, and apoptosis.Ren Fail. 2019 Nov;41(1):750-761. doi: 10.1080/0886022X.2019.1643737. Ren Fail. 2019. PMID: 31441362 Free PMC article.
-
Anti-diabetic and renoprotective effects of aliskiren in streptozotocin-induced diabetic nephropathy in female rats.Naunyn Schmiedebergs Arch Pharmacol. 2016 Dec;389(12):1315-1324. doi: 10.1007/s00210-016-1299-2. Epub 2016 Sep 9. Naunyn Schmiedebergs Arch Pharmacol. 2016. PMID: 27612855
-
Targeting Mitochondria and Reactive Oxygen Species-Driven Pathogenesis in Diabetic Nephropathy.Rev Diabet Stud. 2015 Spring-Summer;12(1-2):134-56. doi: 10.1900/RDS.2015.12.134. Epub 2015 Aug 10. Rev Diabet Stud. 2015. PMID: 26676666 Free PMC article. Review.
-
Endoplasmic Reticulum Stress in Diabetic Nephrology: Regulation, Pathological Role, and Therapeutic Potential.Oxid Med Cell Longev. 2021 Aug 2;2021:7277966. doi: 10.1155/2021/7277966. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34394833 Free PMC article. Review.
Cited by
-
Pharmacotherapy to delay the progression of diabetic kidney disease in people with type 2 diabetes: past, present and future.Ther Adv Endocrinol Metab. 2022 Mar 4;13:20420188221081601. doi: 10.1177/20420188221081601. eCollection 2022. Ther Adv Endocrinol Metab. 2022. PMID: 35281302 Free PMC article. Review.
-
Burdock Fructooligosaccharide Attenuates High Glucose-Induced Apoptosis and Oxidative Stress Injury in Renal Tubular Epithelial Cells.Front Pharmacol. 2021 Dec 9;12:784187. doi: 10.3389/fphar.2021.784187. eCollection 2021. Front Pharmacol. 2021. PMID: 34955856 Free PMC article.
-
GSDMD-mediated pyroptosis: a critical mechanism of diabetic nephropathy.Expert Rev Mol Med. 2021 Dec 27;23:e23. doi: 10.1017/erm.2021.27. Expert Rev Mol Med. 2021. PMID: 34955116 Free PMC article. Review.
-
Vaccinium myrtillus L. ameliorates diabetic nephropathy via modulating metabolites and gut microbiota in rats.Front Pharmacol. 2025 Apr 8;16:1541947. doi: 10.3389/fphar.2025.1541947. eCollection 2025. Front Pharmacol. 2025. PMID: 40264677 Free PMC article.
-
Therapeutic effect and mechanism of combination therapy with ursolic acid and insulin on diabetic nephropathy in a type I diabetic rat model.Front Pharmacol. 2022 Sep 30;13:969207. doi: 10.3389/fphar.2022.969207. eCollection 2022. Front Pharmacol. 2022. PMID: 36249783 Free PMC article.
References
-
- Zhang M., Feng L., Gu J., et al. The attenuation of Moutan Cortex on oxidative stress for renal injury in AGEs-induced mesangial cell dysfunction and streptozotocin-induced diabetic nephropathy rats. Oxidative Medicine and Cellular Longevity. 2014;2014:13. doi: 10.1155/2014/463815.463815 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

