Melatonin and Caffeine Supplementation Used, Respectively, as Protective and Stimulating Agents in the Cryopreservation of Human Sperm Improves Survival, Viability, and Motility after Thawing compared to Traditional TEST-Yolk Buffer
- PMID: 31781344
- PMCID: PMC6855016
- DOI: 10.1155/2019/6472945
Melatonin and Caffeine Supplementation Used, Respectively, as Protective and Stimulating Agents in the Cryopreservation of Human Sperm Improves Survival, Viability, and Motility after Thawing compared to Traditional TEST-Yolk Buffer
Abstract
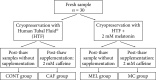
Cryopreservation processes can damage spermatozoa and impair structural and functional cell characteristics. Plasma, nuclear membranes, and cellular organelles can suffer from the freeze and thaw process. This study evaluates the protective and stimulant effect of melatonin and caffeine supplementation on the functional characteristics of human spermatozoa before and after freezing. Thirty seminal samples from normozoospermic men aged 19-45 years old collected between October 2012 and May 2017 were included. Semen samples were supplemented with either 2 mM melatonin (MEL) prior to cryopreservation, 2 mM caffeine (CAF) in postthaw, or CAF and MEL (CM) in precryopreservation and postthaw, respectively. Kinetics and seminal parameters, mitochondrial activity, DNA fragmentation, and reactive oxygen species (ROS) levels were analyzed before and after cryopreservation. A significant reduction in sperm concentration, total and progressive motility, sperm kinetics, and mitochondrial activity, as well as a significant increase in DNA fragmentation and ROS production in postthaw samples compared to fresh samples, was identified. After administration of a caffeine and/or melatonin supplement, there was a significant increase in progressive motility in the CAF (p = 0.005) and CM (p = 0.048) groups, as well as mitochondrial activity in the CM group (p < 0.05). Cryopreservation has negative effects on overall sperm quality and increases ROS production. A combination of caffeine and melatonin in prefreeze and postthaw sperm samples has proven to be a very effective and simple way to improve semen quality. This will be particularly useful for initial low-quality semen samples, those which suffer the most from the freezing/thawing process.
Copyright © 2019 Juliana R. Pariz et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The protective effects of melatonin against cryopreservation-induced oxidative stress in human sperm.Int J Immunopathol Pharmacol. 2015 Mar;28(1):69-76. doi: 10.1177/0394632015572080. Int J Immunopathol Pharmacol. 2015. PMID: 25816408
-
Melatonin affects membrane integrity, intracellular reactive oxygen species, caspase3 activity and AKT phosphorylation in frozen thawed human sperm.Cell Tissue Res. 2018 Apr;372(1):149-159. doi: 10.1007/s00441-017-2743-4. Epub 2017 Dec 1. Cell Tissue Res. 2018. PMID: 29196809
-
Melatonin production improves Senegalese sole sperm motility at night, but fails as a supplement during cryopreservation.Cryobiology. 2024 Dec;117:104974. doi: 10.1016/j.cryobiol.2024.104974. Epub 2024 Sep 26. Cryobiology. 2024. PMID: 39271098
-
The efficacy of antioxidants in sperm parameters and production of reactive oxygen species levels during the freeze-thaw process: A systematic review and meta-analysis.Andrologia. 2020 Apr;52(3):e13514. doi: 10.1111/and.13514. Epub 2020 Jan 21. Andrologia. 2020. PMID: 31967363
-
The Role of Melatonin to Ameliorate Oxidative Stress in Sperm Cells.Int J Mol Sci. 2023 Oct 11;24(20):15056. doi: 10.3390/ijms242015056. Int J Mol Sci. 2023. PMID: 37894737 Free PMC article. Review.
Cited by
-
Coping with Oxidative Stress in Reproductive Pathophysiology and Assisted Reproduction: Melatonin as an Emerging Therapeutical Tool.Antioxidants (Basel). 2022 Dec 30;12(1):86. doi: 10.3390/antiox12010086. Antioxidants (Basel). 2022. PMID: 36670948 Free PMC article. Review.
-
Abusive use of anabolic androgenic steroids, male sexual dysfunction and infertility: an updated review.Front Toxicol. 2024 Apr 22;6:1379272. doi: 10.3389/ftox.2024.1379272. eCollection 2024. Front Toxicol. 2024. PMID: 38711907 Free PMC article. Review.
-
Andrology laboratory techniques for micro-TESE/IVF/ICSI: a narrative review.Asian J Androl. 2025 May 1;27(3):383-391. doi: 10.4103/aja2024122. Epub 2025 Mar 18. Asian J Androl. 2025. PMID: 40101127 Free PMC article. Review.
-
Application of Nanoparticles and Melatonin for Cryopreservation of Gametes and Embryos.Curr Issues Mol Biol. 2022 Sep 5;44(9):4028-4044. doi: 10.3390/cimb44090276. Curr Issues Mol Biol. 2022. PMID: 36135188 Free PMC article. Review.
-
Extend the Survival of Human Sperm In Vitro in Non-Freezing Conditions: Damage Mechanisms, Preservation Technologies, and Clinical Applications.Cells. 2022 Sep 12;11(18):2845. doi: 10.3390/cells11182845. Cells. 2022. PMID: 36139420 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

