Cross-Talk between Oxidative Stress and Inflammation in Preeclampsia
- PMID: 31781353
- PMCID: PMC6875353
- DOI: 10.1155/2019/8238727
Cross-Talk between Oxidative Stress and Inflammation in Preeclampsia
Abstract
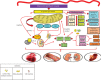
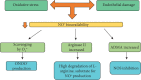
The occurrence of hypertensive syndromes during pregnancy leads to high rates of maternal-fetal morbidity and mortality. Amongst them, preeclampsia (PE) is one of the most common. This review aims to describe the relationship between oxidative stress and inflammation in PE, aiming to reinforce its importance in the context of the disease and to discuss perspectives on clinical and nutritional treatment, in this line of research. Despite the still incomplete understanding of the pathophysiology of PE, it is well accepted that there are placental changes in pregnancy, associated with an imbalance between the production of reactive oxygen species and the antioxidant defence system, characterizing the placental oxidative stress that leads to an increase in the production of proinflammatory cytokines. Hence, a generalized inflammatory process occurs, besides the presence of progressive vascular endothelial damage, leading to the dysfunction of the placenta. There is no consensus in the literature on the best strategies for prevention and treatment of the disease, especially for the control of oxidative stress and inflammation. In view of the above, it is evident the important connection between oxidative stress and inflammatory process in the pathogenesis of PE, being that this disease is capable of causing serious implications on both maternal and fetal health. Reports on the use of anti-inflammatory and antioxidant compounds are analysed and still considered controversial. As such, the field is open for new basic and clinical research, aiming the development of innovative therapeutic approaches to prevent and to treat PE.
Copyright © 2019 Marilene Brandão Tenório et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- World Health Organization. Recommendations of WHO for the Prevention and Treatment of Pre-Eclampsia and Eclampsia – Implications and Actions. Geneva, Switzerland: WHO; 2013.
-
- Malachias M. V. B., Souza W. K. S. B., Plavnik F. L., et al. 7th Brazilian direction of hypertension. Brazilian Archives of Cardiology. 2016;107:1–83.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

