Mechanism Underlying Selective Albuminuria in Minimal Change Nephrotic Syndrome
- PMID: 31781392
- PMCID: PMC6874928
- DOI: 10.1155/2019/5859102
Mechanism Underlying Selective Albuminuria in Minimal Change Nephrotic Syndrome
Abstract
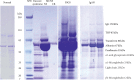
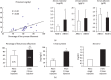
As water and solutes are filtered through the slit membrane, it is an a priori concept that a slit membrane is an essential filtration barrier for proteins, including albumin. However, in cases of minimal change nephrotic syndrome, the number of slit membranes is reduced by the foot process effacement and tight junction-like cell adhesion. Furthermore, albumin endocytosis is enhanced in the podocytes under condition of minimal change disease, and albumin is selectively transported by the albumin receptor FcRn. Suppressing the endocytosis of albumin with anti-FcRn antibody decreases the urinary protein level. The expression of motor molecules, such as cytoplasmic dynein 1 and myosin IX, is increased in the podocytes under conditions of minimal change nephrotic syndrome, suggesting the enhanced transport of vesicles containing albumin. Podocyte vesicle transport may play an important role in the pathology of selective albuminuria in cases of nephrotic syndrome.
Copyright © 2019 Akihiro Tojo.
Conflict of interest statement
The authors declare that they have no conflicts of interest relevant to this work.
Figures




Similar articles
-
Decreased Podocyte Vesicle Transcytosis and Albuminuria in APC C-Terminal Deficiency Mice with Puromycin-Induced Nephrotic Syndrome.Int J Mol Sci. 2021 Dec 14;22(24):13412. doi: 10.3390/ijms222413412. Int J Mol Sci. 2021. PMID: 34948207 Free PMC article.
-
Selective albuminuria via podocyte albumin transport in puromycin nephrotic rats is attenuated by an inhibitor of NADPH oxidase.Kidney Int. 2011 Dec;80(12):1328-38. doi: 10.1038/ki.2011.282. Epub 2011 Aug 17. Kidney Int. 2011. PMID: 21849973
-
Enhanced podocyte vesicle transport in the nephrotic rat.Med Mol Morphol. 2017 Jun;50(2):86-93. doi: 10.1007/s00795-016-0151-6. Epub 2017 Mar 17. Med Mol Morphol. 2017. PMID: 28314927
-
Endocytic Trafficking at the Mature Podocyte Slit Diaphragm.Front Pediatr. 2017 Feb 24;5:32. doi: 10.3389/fped.2017.00032. eCollection 2017. Front Pediatr. 2017. PMID: 28286744 Free PMC article. Review.
-
A Review of Podocyte Biology.Am J Nephrol. 2018;47 Suppl 1:3-13. doi: 10.1159/000481633. Epub 2018 May 31. Am J Nephrol. 2018. PMID: 29852492 Review.
Cited by
-
Decreased Podocyte Vesicle Transcytosis and Albuminuria in APC C-Terminal Deficiency Mice with Puromycin-Induced Nephrotic Syndrome.Int J Mol Sci. 2021 Dec 14;22(24):13412. doi: 10.3390/ijms222413412. Int J Mol Sci. 2021. PMID: 34948207 Free PMC article.
-
Traditional Chinese Medicine in Treating Primary Podocytosis: From Fundamental Science to Clinical Research.Front Pharmacol. 2022 Aug 8;13:932739. doi: 10.3389/fphar.2022.932739. eCollection 2022. Front Pharmacol. 2022. PMID: 36003509 Free PMC article. Review.
-
Oxidized Albumin as a Mediator of Kidney Disease.Antioxidants (Basel). 2021 Mar 8;10(3):404. doi: 10.3390/antiox10030404. Antioxidants (Basel). 2021. PMID: 33800425 Free PMC article. Review.
-
Outcomes of minimal change disease without nephrotic range proteinuria.PLoS One. 2023 Aug 17;18(8):e0289870. doi: 10.1371/journal.pone.0289870. eCollection 2023. PLoS One. 2023. PMID: 37590275 Free PMC article.
-
Effect of proteinuria at relapse on shear wave velocity assessed using ultrasound elastography in children with idiopathic nephrotic syndrome.J Med Ultrason (2001). 2024 Jul;51(3):491-496. doi: 10.1007/s10396-024-01455-7. Epub 2024 Apr 13. J Med Ultrason (2001). 2024. PMID: 38613718
References
-
- Carroll M. F., Temte J. L. Proteinuria in adults: a diagnostic approach. American Family Physician. 2000;62(6):1333–1340. - PubMed
-
- Norden A. G., Lapsley M., Igarashi T., et al. Urinary megalin deficiency implicates abnormal tubular endocytic function in Fanconi syndrome. Journal of the American Society of Nephrology. 2002;13:125–133. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

