Definition of the Anti-inflammatory Oligosaccharides Derived From the Galactosaminogalactan (GAG) From Aspergillus fumigatus
- PMID: 31781511
- PMCID: PMC6851199
- DOI: 10.3389/fcimb.2019.00365
Definition of the Anti-inflammatory Oligosaccharides Derived From the Galactosaminogalactan (GAG) From Aspergillus fumigatus
Abstract
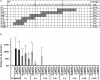
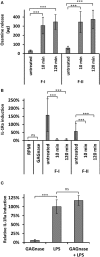
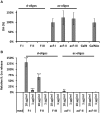
Galactosaminogalactan (GAG) is an insoluble aminosugar polymer produced by Aspergillus fumigatus and has anti-inflammatory properties. Here, the minimum glycosidic sequences required for the induction of IL-1Ra by peripheral blood mononuclear cells (PBMCs) was investigated. Using chemical degradation of native GAG to isolate soluble oligomers, we have found that the de-N-acetylation of galactosamine residues and the size of oligomer are critical for the in vitro immune response. A minimal oligomer size of 20 galactosamine residues is required for the anti-inflammatory response but the presence of galactose residues is not necessary. In a Dextran sulfate induced colitis mouse model, a fraction of de-N-acetylated oligomers of 13 < dp < 20 rescue inflammatory damage like the native GAG polymer in an IL-1Ra dependent pathway. Our results demonstrate the therapeutic suitability of water-soluble GAG oligosaccharides in IL-1 mediated hyper-inflammatory diseases and suggest that α-1,4-galactosamine oligomers chemically synthesized could represent new anti-inflammatory glycodrugs.
Keywords: Aspergillus fumigatus; IL-Ra; anti-inflammatory response; galactosaminogalactan; glycodrug.
Copyright © 2019 Gressler, Heddergott, N'Go, Renga, Oikonomou, Moretti, Coddeville, Gaifem, Silvestre, Romani, Latgé and Fontaine.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

