A Supramolecular Interaction of a Ruthenium Complex With Calf-Thymus DNA: A Ligand Binding Approach by NMR Spectroscopy
- PMID: 31781544
- PMCID: PMC6857657
- DOI: 10.3389/fchem.2019.00762
A Supramolecular Interaction of a Ruthenium Complex With Calf-Thymus DNA: A Ligand Binding Approach by NMR Spectroscopy
Abstract
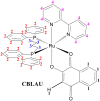
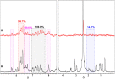
Lawsone itself exhibits interesting biological activities, and its complexation with a metal center can improve the potency. In this context a cytotoxic Ru-complex, [Ru(law)(dppb)(bipy)] (law = lawsone, dppb = 1,4-bis(diphenylphosphino)butane and bipy = 2,2'-bipyridine), named as CBLAU, was prepared as reported. In this work, NMR binding-target studies were performed to bring to light the most accessible interaction sites of this Ru-complex toward Calf-Thymus DNA (CT-DNA, used as a model), in a similar approach used for other metallic complexes with anti-cancer activity, such as cisplatin and carboplatin. Advanced and robust NMR binding-target studies, among them Saturation Transfer Difference (STD)-NMR and longitudinal relaxometry (T1), were explored. The 1H and 31P -NMR data indicate that the structure of Ru-complex remains preserved in the presence of CT-DNA, and some linewidth broadening is also observed for all the signals, pointing out some interaction. Looking at the binding efficiency, the T1 values are highly influenced by the formation of the CBLAU-DNA adduct, decreasing from 11.4 s (without DNA) to 1.4 s (with DNA), where the difference is bigger for the lawsone protons. Besides, the STD-NMR titration experiments revealed a stronger interaction (KD = 5.9 mM) for CBLAU-DNA in comparison to non-complexed lawsone-DNA (KD = 34.0 mM). The epitope map, obtained by STD-NMR, shows that aromatic protons from the complexed lawsone exhibits higher saturation transfer, in comparison to other Ru-ligands (DPPB and bipy), suggesting the supramolecular contact with CT-DNA takes place by the lawsone face of the Ru-complex, possibly by a spatial π-π stacking involving π-bonds on nucleic acids segments of the DNA chain and the naphthoquinone group.
Keywords: CT-DNA; NMR; anti-cancer; binding interactions; lawsone; ruthenium complex.
Copyright © 2019 Kock, Costa, de Oliveira, Batista, Ferreira and Venâncio.
Figures





Similar articles
-
Ru(II)-Naphthoquinone complexes with high selectivity for triple-negative breast cancer.Dalton Trans. 2020 Nov 25;49(45):16193-16203. doi: 10.1039/d0dt01091j. Dalton Trans. 2020. PMID: 32329497
-
Non-mutagenic Ru(ii) complexes: cytotoxicity, topoisomerase IB inhibition, DNA and HSA binding.Dalton Trans. 2019 Oct 7;48(39):14885-14897. doi: 10.1039/c9dt01905g. Dalton Trans. 2019. PMID: 31555783
-
Antiparasitic activities of novel ruthenium/lapachol complexes.J Inorg Biochem. 2014 Jul;136:33-9. doi: 10.1016/j.jinorgbio.2014.03.009. Epub 2014 Mar 27. J Inorg Biochem. 2014. PMID: 24727183
-
Correlation Between Molecular Modelling and Spectroscopic Techniques in Investigation With DNA Binding Interaction of Ruthenium(II) Complexes.J Fluoresc. 2017 Mar;27(2):587-594. doi: 10.1007/s10895-016-1986-x. Epub 2016 Dec 6. J Fluoresc. 2017. PMID: 27924438 Review.
-
Critical Overview of the Use of Ru(II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy.Acc Chem Res. 2017 Nov 21;50(11):2727-2736. doi: 10.1021/acs.accounts.7b00180. Epub 2017 Oct 23. Acc Chem Res. 2017. PMID: 29058879 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

