Non-invasive Production of Multi-Compartmental Biodegradable Polymer Microneedles for Controlled Intradermal Drug Release of Labile Molecules
- PMID: 31781550
- PMCID: PMC6856554
- DOI: 10.3389/fbioe.2019.00296
Non-invasive Production of Multi-Compartmental Biodegradable Polymer Microneedles for Controlled Intradermal Drug Release of Labile Molecules
Abstract
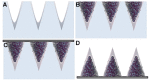
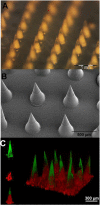
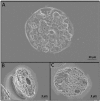
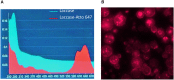
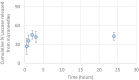
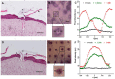
Transdermal drug delivery represents an appealing alternative to conventional drug administration systems. In fact, due to their high patient compliance, the development of dissolvable and biodegradable polymer microneedles has recently attracted great attention. Although stamp-based procedures guarantee high tip resolution and reproducibility, they have long processing times, low levels of system engineering, are a source of possible contaminants, and thermo-sensitive drugs cannot be used in conjunction with them. In this work, a novel stamp-based microneedle fabrication method is proposed. It provides a rapid room-temperature production of multi-compartmental biodegradable polymeric microneedles for controlled intradermal drug release. Solvent casting was carried out for only a few minutes and produced a short dissolvable tip made of polyvinylpyrrolidone (PVP). The rest of the stamp was then filled with degradable poly(lactide-co-glycolide) (PLGA) microparticles (μPs) quickly compacted with a vapor-assisted plasticization. The outcome was an array of microneedles with tunable release. The ability of the resulting microneedles to indent was assessed using pig cadaver skin. Controlled intradermal delivery was demonstrated by loading both the tip and the body of the microneedles with model therapeutics; POXA1b laccase from Pleurotus ostreatus is a commercial enzyme used for the whitening of skin spots. The action and indentation of the enzyme-loaded microneedle action were assessed in an in vitro skin model and this highlighted their ability to control the kinetic release of the encapsulated compound.
Keywords: controlled release; enzyme; multi-compartmental; polymer microneedles; skin model.
Copyright © 2019 Battisti, Vecchione, Casale, Pennacchio, Lettera, Jamaledin, Profeta, Di Natale, Imparato, Urciuolo and Netti.
Figures





References
-
- Bronaugh R., Maibach H. (1999). Percutaneous absorption drug-cosmetics-mechanism-methodology. J. Appl. Cosmetol. 17, 116–119.
LinkOut - more resources
Full Text Sources

