Serum Levels of BAFF and APRIL Predict Clinical Response in Anti-PLA2R-Positive Primary Membranous Nephropathy
- PMID: 31781684
- PMCID: PMC6874868
- DOI: 10.1155/2019/8483650
Serum Levels of BAFF and APRIL Predict Clinical Response in Anti-PLA2R-Positive Primary Membranous Nephropathy
Abstract
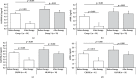
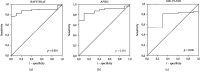
Primary membranous nephropathy (PMN) is a renal-specific autoimmune disease caused by circulating autoantibodies that target glomerular podocyte antigens (PLA2R/THSD7A). However, very little is known on the molecular mechanisms controlling B cell response in this nephropathy. The present study was aimed at correlating the serum levels of B cell activators BAFF/BLyS and APRIL with the presence of anti-PLA2R antibodies in PMN patients and with long-term clinical outcome. To this aim, 51 patients with anti-PLA2R-positive biopsy-proven PMN and nephrotic range proteinuria (>3.5 g/24 hours) were enrolled between January 2009 and December 2015 and treated with conventional 6-month immunosuppressive therapy. After 6 months, 29 patients (56.9%) cleared circulating anti-PLA2R, while in remaining 22 (43.1%), they persisted. Intriguingly, in the first group, baseline serum levels of BAFF/BLyS and APRIL were significantly lower than those in the second one. Moreover, after 6 months of immunosuppressive therapy, an overall reduction in both cytokine serum levels was observed. However, in PMN patients with anti-PLA2R clearance, this reduction was more prominent, as compared with those with anti-PLA2R persistence. When related to clinical outcome, lower baseline BAFF/BLyS (<6.05 ng/mL) and APRIL (<4.20 ng/mL) serum levels were associated with significantly higher probability to achieve complete or partial remission after 24-month follow-up. After dividing the entire study cohort into three groups depending on both cytokine baseline serum levels, patients with both BAFF/BLyS and APRIL below the cut-off showed a significantly higher rate of complete or partial remission as compared with patients with only one cytokine above the cut-off, while the composite endpoint was achieved in a very low rate of patients with both cytokines above the cut-off. Taken together, these results provide new insights into the role of BAFF/BLyS and APRIL in both the pathogenesis of anti-PLA2R-positive PMN and the response to immunosuppressive therapy.
Copyright © 2019 Giuseppe Stefano Netti et al.
Conflict of interest statement
The authors have nothing to disclose.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

