Engagement in Care, Viral Suppression, Drug Resistance, and Reasons for Nonengagement After Home-Based Same-Day Antiretroviral Therapy Initiation in Lesotho: A Two-Year Follow-up of the CASCADE Trial
- PMID: 31781759
- PMCID: PMC7745003
- DOI: 10.1093/cid/ciz1126
Engagement in Care, Viral Suppression, Drug Resistance, and Reasons for Nonengagement After Home-Based Same-Day Antiretroviral Therapy Initiation in Lesotho: A Two-Year Follow-up of the CASCADE Trial
Abstract
Background: The CASCADE trial showed that compared with usual care (UC), offering same-day (SD) antiretroviral therapy (ART) during home-based human immunodeficiency virus testing improved engagement in care and viral suppression 12 months after diagnosis. However, questions remain regarding long-term outcomes and the risk of propagating drug resistance.
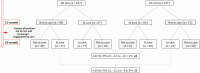
Methods: After completion of the primary endpoint at 12 months, participants not in care in both arms were traced and encouraged to access care. At 24 months, the following outcomes were assessed in both arms: engagement in care, viral suppression, and reasons for nonengagement. Furthermore, we explored the acquisition of drug resistance mutations (DRMs) among SD arm nonlinkers.
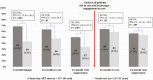
Results: At 24 months, 64% (88/137) in the SD arm vs 59% (81/137) in the UC arm were in care (absolute difference [AD], 5%; 95% confidence interval [CI], -6 to16; P = .38) and 57% (78/137) vs 54% (74/137) had documented viral suppression (AD, 3%; 95% CI, -9 to 15; P = .28). Among 36 participants alive and not in care at 24 months with ascertained status, the majority rejected contact with the health system or were unwilling to take ART. Among 8 interviewed SD arm nonlinkers, 6 had not initiated ART upon enrollment, and no acquired DRMs were detected. Two had taken the initial 30-day ART supply and acquired DRMs.
Conclusions: SD ART resulted in higher rates of engagement in care and viral suppression at 12 months but not at 24 months. Leveling off between both arms was driven by linkage beyond 12 months in the UC arm. We did not observe compensatory long-term disengagement in the SD arm. These long-term results endorse SD ART initiation policies.
Clinical trials registration: NCT02692027.
Keywords: HIV infection; Lesotho; rapid ART initiation; retention in care; same-day ART.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
Necessary but Not Sufficient: The Need for Innovative Strategies to Enhance Retention and Viral Suppression After Rapid Initiation of Antiretroviral Therapy.Clin Infect Dis. 2020 Dec 17;71(10):2615-2617. doi: 10.1093/cid/ciz1176. Clin Infect Dis. 2020. PMID: 31811286 Free PMC article. No abstract available.
References
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: what’s new. WHO: Geneva, Switzerland: World Health Organization, 2015. Available at: https://apps.who.int/iris/bitstream/handle/10665/198064/9789241509893_en.... Accessed 21 October 2019.
-
- Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS 2012; 26:2059–67. - PubMed

