Role of the Gonococcal Neisserial Heparin Binding Antigen in Microcolony Formation, and Serum Resistance and Adherence to Epithelial Cells
- PMID: 31781772
- PMCID: PMC7184908
- DOI: 10.1093/infdis/jiz628
Role of the Gonococcal Neisserial Heparin Binding Antigen in Microcolony Formation, and Serum Resistance and Adherence to Epithelial Cells
Abstract
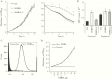
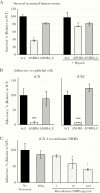
The sexually transmitted infection gonorrhoea is on the rise worldwide and an increased understanding of the mechanisms of colonization and pathogenesis of Neisseria gonorrhoeae is required to aid development of new treatment and prevention strategies. In the current study, we investigate the neisserial heparin-binding antigen (NHBA) of N. gonorrhoeae and confirm its role in binding to several glycans, including heparin, and identify interactions of NHBA with both gonococcal and host cells. Furthermore, we report that a gonococcal nhba mutant displays decreased cell aggregation and microcolony formation, as well as reduced survival in human serum and reduced adherence to human cervical and urethral epithelial cells, relative to the wild-type strain. These data indicate that the gonococcal NHBA contributes to several aspects of the colonization and survival of N. gonorrhoeae and may be a target for new antimicrobial or vaccines.
Keywords: Neisseria gonorrhoeae; NHBA; adherence; aggregation; glycan; gonococcus; heparin; microcolony; neisserial heparin-binding Antigen; serum resistance; vaccine antigen.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures




Similar articles
-
Glycointeractome of Neisseria gonorrhoeae: Identification of Host Glycans Targeted by the Gonococcus To Facilitate Adherence to Cervical and Urethral Epithelial Cells.mBio. 2019 Jul 9;10(4):e01339-19. doi: 10.1128/mBio.01339-19. mBio. 2019. PMID: 31289181 Free PMC article.
-
The Serogroup B Meningococcal Vaccine Bexsero Elicits Antibodies to Neisseria gonorrhoeae.Clin Infect Dis. 2019 Sep 13;69(7):1101-1111. doi: 10.1093/cid/ciy1061. Clin Infect Dis. 2019. PMID: 30551148 Free PMC article.
-
Neisserial Heparin Binding Antigen (NHBA) Contributes to the Adhesion of Neisseria meningitidis to Human Epithelial Cells.PLoS One. 2016 Oct 25;11(10):e0162878. doi: 10.1371/journal.pone.0162878. eCollection 2016. PLoS One. 2016. PMID: 27780200 Free PMC article.
-
The sweet side of the pathogenic Neisseria: the role of glycan interactions in colonisation and disease.Pathog Dis. 2017 Jul 31;75(5):ftx063. doi: 10.1093/femspd/ftx063. Pathog Dis. 2017. PMID: 28633281 Free PMC article. Review.
-
Neisseria proteomics for antigen discovery and vaccine development.Expert Rev Proteomics. 2014 Oct;11(5):573-91. doi: 10.1586/14789450.2014.938640. Epub 2014 Jul 12. Expert Rev Proteomics. 2014. PMID: 25017717 Review.
Cited by
-
Glycan-mediated adhesion mechanisms in antibiotic-resistant bacteria.BBA Adv. 2025 Mar 14;7:100156. doi: 10.1016/j.bbadva.2025.100156. eCollection 2025. BBA Adv. 2025. PMID: 40207210 Free PMC article.
-
The serogroup B meningococcal outer membrane vesicle-based vaccine 4CMenB induces cross-species protection against Neisseria gonorrhoeae.PLoS Pathog. 2020 Dec 8;16(12):e1008602. doi: 10.1371/journal.ppat.1008602. eCollection 2020 Dec. PLoS Pathog. 2020. PMID: 33290434 Free PMC article.
-
The Neisseria gonorrhoeae Vaccine Candidate NHBA Elicits Antibodies That Are Bactericidal, Opsonophagocytic and That Reduce Gonococcal Adherence to Epithelial Cells.Vaccines (Basel). 2020 May 13;8(2):219. doi: 10.3390/vaccines8020219. Vaccines (Basel). 2020. PMID: 32414194 Free PMC article.
-
Neisseria gonorrhoeae vaccines: a contemporary overview.Clin Microbiol Rev. 2024 Mar 14;37(1):e0009423. doi: 10.1128/cmr.00094-23. Epub 2024 Jan 16. Clin Microbiol Rev. 2024. PMID: 38226640 Free PMC article. Review.
-
Infection With the US Neisseria meningitidis Urethritis Clade Does Not Lower Future Risk of Urethral Gonorrhea.Clin Infect Dis. 2022 Jul 6;74(12):2159-2165. doi: 10.1093/cid/ciab824. Clin Infect Dis. 2022. PMID: 34543381 Free PMC article.
References
-
- World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections—2008. Geneva, Switzerland: World Health Organization, 2012.
-
- Hook EW, Handsfield HH. Gonococcal infection in the adult. In: Holmes KK, ed. Sexually transmitted diseases. New York, NY: McGraw-Hill, 2008:627–45.
-
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf Accessed 8 April 2018.
-
- World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics http://www.who.int/medicines/publications/global-priority-list-antibioti.... Accessed 8 April 2018.

