Serotonin uptake via plasma membrane monoamine transporter during myocardial ischemia-reperfusion in the rat heart in vivo
- PMID: 31782271
- PMCID: PMC6882957
- DOI: 10.14814/phy2.14297
Serotonin uptake via plasma membrane monoamine transporter during myocardial ischemia-reperfusion in the rat heart in vivo
Abstract
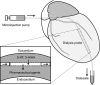
Serotonin (5-HT) accumulates in the heart during myocardial ischemia and induces deleterious effects on the cardiomyocytes through receptor-dependent and monoamine oxidase-dependent pathways. We aimed to clarify the involvement of extra-neuronal monoamine transporters in the clearance of 5-HT during ischemia and reperfusion in the heart. Using a microdialysis technique in the anesthetized Wistar rat heart, we monitored myocardial interstitial 5-HT and 5-hydroxyindole acetic acid (5-HIAA) concentration by means of electro-chemical detection coupled with high-performance liquid chromatography (HPLC-ECD). Effects of inhibitors of the plasma membrane monoamine transporter (PMAT) and the organic cation transporter 3 (OCT3) (decynium-22 and corticosterone) on the 5-HT and 5-HIAA concentrations during baseline, coronary occlusion, and reperfusion were investigated. Basal dialysate 5-HT concentration were increased by local administration of decynium-22, but not by corticosterone. Addition of fluoxetine, a serotonin transporter (SERT) inhibitor further increased the 5-HT concentration upon during administration of decynium-22. Decynium-22 elevated the background level of 5-HT during coronary occlusion and maintained 5-HT concentration at a high level during reperfusion. Production of 5-HIAA in the early reperfusion was significantly suppressed by decynium-22. These results indicate that PMAT and SERT independently regulate basal level of interstitial 5-HT, and PMAT plays a more important role in the clearance of 5-HT during reperfusion. These data suggest the involvement of PMAT in the monoamine oxidase-dependent deleterious pathway in the heart.
Keywords: 5-HT; in vivo cardiac microdialysis; ischemia reperfusion injury; organic cation transporter; plasma membrane monoamine transporter; serotonin transporter.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
No conflicts of interest, financial, or otherwise, are declared by the authors.
Figures




References
-
- Akiyama, T. , Yamazaki, T. , & Ninomiya, I. (1991). In vivo monitoring of myocardial interstitial norepinephrine by dialysis technique. American Journal of Physiology, 261, H1643–H1647. - PubMed
-
- Barnes, K. , Dobrzynski, H. , Foppolo, S. , Beal, P. R. , Ismat, F. , Scullion, E. R. , … Baldwin, S. A. (2006). Distribution and functional characterization of equilibrative nucleoside transporter‐4, a novel cardiac adenosine transporter activated at acidic pH. Circulation Research, 99, 510–519. - PubMed
-
- Bianchi, P. , Kunduzova, O. , Masini, E. , Cambon, C. , Bani, D. , Raimondi, L. , … Parini, A. (2005). Oxidative stress by monoamine oxidase mediates receptor‐independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation, 112, 3297–3305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

