Increase of Dose Associated With Decrease in Protection Against Controlled Human Malaria Infection by PfSPZ Vaccine in Tanzanian Adults
- PMID: 31782768
- PMCID: PMC7947995
- DOI: 10.1093/cid/ciz1152
Increase of Dose Associated With Decrease in Protection Against Controlled Human Malaria Infection by PfSPZ Vaccine in Tanzanian Adults
Abstract
Background: A vaccine would be an ideal tool for reducing malaria's impact. PfSPZ Vaccine (radiation attenuated, aseptic, purified, cryopreserved Plasmodium falciparum [Pf] sporozoites [SPZ]) has been well tolerated and safe in >1526 malaria-naive and experienced 6-month to 65-year-olds in the United States, Europe, and Africa. When vaccine efficacy (VE) of 5 doses of 2.7 × 105 PfSPZ of PfSPZ Vaccine was assessed in adults against controlled human malaria infection (CHMI) in the United States and Tanzania and intense field transmission of heterogeneous Pf in Mali, Tanzanians had the lowest VE (20%).
Methods: To increase VE in Tanzania, we increased PfSPZ/dose (9 × 105 or 1.8 × 106) and decreased numbers of doses to 3 at 8-week intervals in a double blind, placebo-controlled trial.
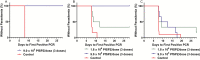
Results: All 22 CHMIs in controls resulted in parasitemia by quantitative polymerase chain reaction. For the 9 × 105 PfSPZ group, VE was 100% (5/5) at 3 or 11 weeks (P < .000l, Barnard test, 2-tailed). For 1.8 × 106 PfSPZ, VE was 33% (2/6) at 7.5 weeks (P = .028). VE of dosage groups (100% vs 33%) was significantly different (P = .022). Volunteers underwent repeat CHMI at 37-40 weeks after last dose. 6/6 and 5/6 volunteers developed parasitemia, but time to first parasitemia was significantly longer than controls in the 9 × 105 PfSPZ group (10.89 vs 7.80 days) (P = .039), indicating a significant reduction in parasites in the liver. Antibody and T-cell responses were higher in the 1.8 × 106 PfSPZ group.
Conclusions: In Tanzania, increasing the dose from 2.7 × 105 to 9 × 105 PfSPZ increased VE from 20% to 100%, but increasing to 1.8 × 106 PfSPZ significantly reduced VE.
Clinical trials registration: NCT02613520.
Keywords: Plasmodium falciparum; PfSPZ; controlled human malaria infection; malaria; vaccine efficacy.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


References
-
- World Health Organization. Global Malaria Programme. World malaria report 2015. Geneva: World Health Organization, 2015.
-
- World Health Organization. World malaria report 2018. Geneva: World Health Organization, 2018.
-
- Epstein JE, Tewari K, Lyke KE, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+T cell immunity. Science 2011; 334:475–80. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

