UHRF1 downmodulation enhances antitumor effects of histone deacetylase inhibitors in retinoblastoma by augmenting oxidative stress-mediated apoptosis
- PMID: 31782885
- PMCID: PMC6998393
- DOI: 10.1002/1878-0261.12607
UHRF1 downmodulation enhances antitumor effects of histone deacetylase inhibitors in retinoblastoma by augmenting oxidative stress-mediated apoptosis
Abstract
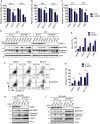
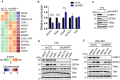
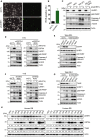
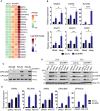
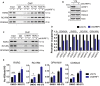
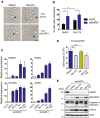
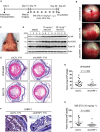
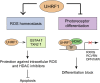
Identification of new genetic pathways or molecular targets that sensitize cancer cells to chemotherapeutic drugs may improve the efficacy of current chemotherapy. Here, we report that downmodulation of UHRF1 (ubiquitin-like with PHD and RING finger domains 1) in retinoblastoma (RB) cells increases the sensitivity to histone deacetylase (HDAC) inhibitors, augmenting apoptotic cell death. We found that UHRF1 depletion downregulates two redox-responsive genes GSTA4 (glutathione S-transferase α4) and TXN2 (thioredoxin-2) in RB cells, and increases the basal level of intracellular oxidative stress. Antioxidant treatment significantly reduced both basal and HDAC inhibitor-induced DNA damage and apoptosis in UHRF1-depleted cells. Knockdown of GSTA4 or TXN2 sensitized RB cells to HDAC inhibitors, demonstrating that GSTA4 and TXN2 play key roles in redox homeostasis in RB cells and the susceptibility to HDAC inhibitor treatment upon UHRF1 depletion. In human primary RB, GSTA4 and TXN2 proteins were found to be mostly elevated along with high UHRF1 expression. In addition to augmentation of apoptosis in UHRF1-depleted RB cells, we also show that UHRF1 downmodulation derepresses the expression of photoreceptor-specific genes in RB cells in cooperation with a HDAC inhibitor MS-275 and promotes neuron-like differentiation. However, further investigation revealed that the enhanced growth-inhibitory effects of MS-275 in UHRF1-depleted cells were still mainly due to robust apoptosis induction rather than differentiation-mediated growth arrest. Consistent with our findings, UHRF1 depletion in RB cells increased the therapeutic efficacy of MS-275 in murine orthotopic xenografts. These results provide a novel basis for potential benefits of UHRF1 targeting for RB treatment.
Keywords: HDAC inhibitors; UHRF1; chemotherapy; drug sensitivity; retinoblastoma.
© 2019 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bronner C, Krifa M and Mousli M (2013) Increasing role of UHRF1 in the reading and inheritance of the epigenetic code as well as in tumorogenesis. Biochem Pharmacol 86, 1643–1649. - PubMed
-
- Chan HS, Gallie BL, Munier FL and Beck Popovic M (2005) Chemotherapy for retinoblastoma. Ophthalmol Clin North Am 18, 55–63, viii. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

