Orchestrating mitochondria in neurons: Cytoskeleton as the conductor
- PMID: 31782907
- PMCID: PMC7187307
- DOI: 10.1002/cm.21585
Orchestrating mitochondria in neurons: Cytoskeleton as the conductor
Abstract
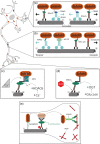
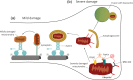
Mitochondria are crucial to support synaptic activity, particularly through ATP production and Ca2+ homeostasis. This implies that mitochondria need to be well distributed throughout the different neuronal sub-compartments. To achieve this, a tight and precise regulation of several neuronal cytoskeleton players is necessary to transport and dock mitochondria. As post-mitotic cells, neurons are highly dependent on mitochondrial quality control mechanisms and several cytoskeleton proteins have been implicated in mitophagy. Therefore, all of these processes are orchestrated by the crosstalk between mitochondria and the neuronal cytoskeleton to form a coordinated and tuned symphony.
Keywords: docking; mitochondria; neuronal cytoskeleton; synapse; transport.
© 2019 The Authors. Cytoskeleton published by Wiley Periodicals, Inc.
Figures
Similar articles
-
Highly Specialized Mechanisms for Mitochondrial Transport in Neurons: From Intracellular Mobility to Intercellular Transfer of Mitochondria.Biomolecules. 2023 Jun 3;13(6):938. doi: 10.3390/biom13060938. Biomolecules. 2023. PMID: 37371518 Free PMC article. Review.
-
Regulation of mitochondrial transport in neurons.Exp Cell Res. 2015 May 15;334(1):35-44. doi: 10.1016/j.yexcr.2015.01.004. Epub 2015 Jan 19. Exp Cell Res. 2015. PMID: 25612908 Free PMC article. Review.
-
Neurons: The Interplay between Cytoskeleton, Ion Channels/Transporters and Mitochondria.Cells. 2022 Aug 11;11(16):2499. doi: 10.3390/cells11162499. Cells. 2022. PMID: 36010576 Free PMC article. Review.
-
Mitochondria on the move.Trends Cell Biol. 2007 Oct;17(10):502-10. doi: 10.1016/j.tcb.2007.07.008. Epub 2007 Sep 4. Trends Cell Biol. 2007. PMID: 17804238 Review.
-
Spatially Stable Mitochondrial Compartments Fuel Local Translation during Plasticity.Cell. 2019 Jan 10;176(1-2):73-84.e15. doi: 10.1016/j.cell.2018.12.013. Epub 2019 Jan 3. Cell. 2019. PMID: 30612742
Cited by
-
Mitochondrial Quality Control Strategies: Potential Therapeutic Targets for Neurodegenerative Diseases?Front Neurosci. 2021 Nov 12;15:746873. doi: 10.3389/fnins.2021.746873. eCollection 2021. Front Neurosci. 2021. PMID: 34867159 Free PMC article. Review.
-
Motor proteins at the mitochondria-cytoskeleton interface.J Cell Sci. 2021 Apr 1;134(7):jcs226084. doi: 10.1242/jcs.226084. Epub 2021 Apr 13. J Cell Sci. 2021. PMID: 33912943 Free PMC article. Review.
-
Intermittent Hypoxia Promotes Functional Neuroprotection from Retinal Ischemia in Untreated First-Generation Offspring: Proteomic Mechanistic Insights.Invest Ophthalmol Vis Sci. 2020 Sep 1;61(11):15. doi: 10.1167/iovs.61.11.15. Invest Ophthalmol Vis Sci. 2020. PMID: 32910134 Free PMC article.
-
Cross Talk at the Cytoskeleton-Plasma Membrane Interface: Impact on Neuronal Morphology and Functions.Int J Mol Sci. 2020 Nov 30;21(23):9133. doi: 10.3390/ijms21239133. Int J Mol Sci. 2020. PMID: 33266269 Free PMC article. Review.
-
Mitochondrial Dynamics and mRNA Translation: A Local Synaptic Tale.Biology (Basel). 2024 Sep 23;13(9):746. doi: 10.3390/biology13090746. Biology (Basel). 2024. PMID: 39336173 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

