Necroptosis in the Pathophysiology of Disease
- PMID: 31783008
- PMCID: PMC6983729
- DOI: 10.1016/j.ajpath.2019.10.012
Necroptosis in the Pathophysiology of Disease
Abstract
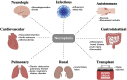
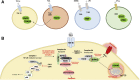
Over the past 15 years, elegant studies have demonstrated that in certain conditions, programed cell death resembles necrosis and depends on a unique molecular pathway with no overlap with apoptosis. This form of regulated necrosis is represented by necroptosis, in which the receptor-interacting protein kinase-3 and its substrate mixed-lineage kinase domain-like protein play a crucial role. With the development of knockout mouse models and molecular inhibitors unique to necroptotic proteins, this cell death has been found to occur in virtually all tissues and diseases evaluated. There are different immunologic consequences depending on whether cells die through apoptosis or necroptosis. Therefore, distinguishing between these two forms of cell death may be crucial during pathologic evaluations. In this review, we provide an understanding of necroptotic cell-death and highlight diseases in which necroptosis has been found to play a role. We also discuss the inhibitors of necroptosis and the ways these inhibitors have been used in preclinical models of diseases. These two discussions offer an understanding of the role of necroptosis in diseases and will foster efforts to pharmacologically target this unique yet pervasive form of programed cell death in the clinic.
Copyright © 2020 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Lau A., Wang S., Jiang J., Haig A., Pavlosky A., Linkermann A., Zhang Z.X., Jevnikar A.M. RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival. Am J Transplant. 2013;13:2805–2818. - PubMed
-
- Kwok C., Pavlosky A., Lian D., Jiang J., Huang X., Yin Z., Liu W., Haig A., Jevnikar A.M., Zhang Z.X. Necroptosis is involved in CD4+T cell-mediated microvascular endothelial cell death and chronic cardiac allograft rejection. Transplantation. 2017;101:2026–2037. - PubMed
-
- Feng S., Yang Y., Mei Y., Ma L., Zhu D.E., Hoti N., Castanares M., Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–2067. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

