1α, 25 Dihydroxyvitamin D (1,25(OH)2D) inhibits the T cell suppressive function of myeloid derived suppressor cells (MDSC)
- PMID: 31783150
- PMCID: PMC8041088
- DOI: 10.1016/j.jsbmb.2019.105557
1α, 25 Dihydroxyvitamin D (1,25(OH)2D) inhibits the T cell suppressive function of myeloid derived suppressor cells (MDSC)
Abstract
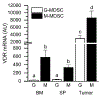
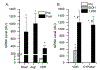
Myeloid derived suppressor cells (MDSC) suppress the ability of cytotoxic T cells to attack and clear tumor cells from the body. The active form of vitamin D, 1,25 dihydroxyvitamin D (1,25(OH)2D), regulates myeloid cell biology and previous research showed that in mouse models 1,25(OH)2D reduced the tumor level of CD34+ cells, an MDSC precursor, and reduced metastasis. We tested whether MDSC are vitamin D target cells by examining granulocytic- (G-MDSC) and monocytic (M-MDSC) MDSC from tumors, spleen, and bone marrow. Vitamin D receptor (VDR) mRNA levels are low in MDSC from bone marrow and spleen but are 20-fold higher in tumor MDSC. At all sites, M-MDSC have 4-fold higher VDR mRNA expression than G-MDSC. Bone marrow MDSC were induced to differentiate in vitro into tumor MDSC-like cells by treating with IFN-γ, IL-13, and GM-CSF for 48 h. This treatment significantly elevated Arg1 and Nos2 levels, activated the T cell-suppressive function of MDSC, increased VDR expression 50-fold, and made the MDSC responsive to 1,25(OH)2D treatment. Importantly, 1,25(OH)2D treatment reduced the T cell suppressive capacity of cytokine-induced total MDSC and M-MDSC by ≥70 % and tumor-derived M-MDSC by 30-50 %. Consistent with this finding, VDR deletion (KO) increased T cell suppressive function of in vitro M-MDSC by 30 % and of tumor-derived M-MDSC by 50 % and G-MDSC by 400 %. VDR KO did not alter Nos2 mRNA levels but significantly increased Arg1 mRNA levels in tumor M-MDSC by 100 %. In contrast, 1,25(OH)2D treatment reduced nitric oxide production in both in vitro derived M- and G- MDSC. The major finding of this study is that 1,25(OH)2D signaling through the VDR decreases the immunosuppressive capability of MDSC. Collectively, our data suggest that activation of vitamin D signaling could be used to suppress MDSC function and release a constraint on T-cell mediated clearance of tumor cells.
Keywords: Cancer; Immunology; Myeloid derived suppressor cell; Vitamin D receptor.
Copyright © 2019. Published by Elsevier Ltd.
Figures
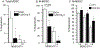
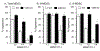
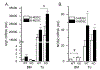
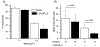
Similar articles
-
Prostaglanin-E2 Potentiates the Suppressive Functions of Human Mononuclear Myeloid-Derived Suppressor Cells and Increases Their Capacity to Expand IL-10-Producing Regulatory T Cell Subsets.Front Immunol. 2019 Mar 18;10:475. doi: 10.3389/fimmu.2019.00475. eCollection 2019. Front Immunol. 2019. PMID: 30936876 Free PMC article.
-
Immunosuppressive effects and mechanisms of three myeloid-derived suppressor cells subsets including monocytic-myeloid-derived suppressor cells, granulocytic-myeloid-derived suppressor cells, and immature-myeloid-derived suppressor cells.J Cancer Res Ther. 2021 Jul-Sep;17(4):1093-1100. doi: 10.4103/jcrt.JCRT_1222_20. J Cancer Res Ther. 2021. PMID: 34528569
-
Effect of calcitriol on myeloid-derived suppressor cells in physiological aging.J Steroid Biochem Mol Biol. 2025 Jul;251:106768. doi: 10.1016/j.jsbmb.2025.106768. Epub 2025 Apr 30. J Steroid Biochem Mol Biol. 2025. PMID: 40316223
-
Adjuvants and myeloid-derived suppressor cells: enemies or allies in therapeutic cancer vaccination.Hum Vaccin Immunother. 2014;10(11):3251-60. doi: 10.4161/hv.29847. Hum Vaccin Immunother. 2014. PMID: 25483674 Free PMC article. Review.
-
The effect of 1,25 dihydroxyvitamin D3 treatment on the mRNA levels of β catenin target genes in mice with colonic inactivation of both APC alleles.J Steroid Biochem Mol Biol. 2015 Apr;148:103-10. doi: 10.1016/j.jsbmb.2015.01.009. Epub 2015 Jan 15. J Steroid Biochem Mol Biol. 2015. PMID: 25597951 Free PMC article. Review.
Cited by
-
Myeloid-Derived Suppressor Cells in Cancer: Mechanistic Insights and Targeted Therapeutic Innovations.MedComm (2020). 2025 May 31;6(6):e70231. doi: 10.1002/mco2.70231. eCollection 2025 Jun. MedComm (2020). 2025. PMID: 40452814 Free PMC article. Review.
-
Nucleic Acid-Based Approaches for Tumor Therapy.Cells. 2020 Sep 9;9(9):2061. doi: 10.3390/cells9092061. Cells. 2020. PMID: 32917034 Free PMC article. Review.
-
Myeloid-Derived Suppressor Cells in COVID-19: The Paradox of Good.Front Immunol. 2022 Apr 27;13:842949. doi: 10.3389/fimmu.2022.842949. eCollection 2022. Front Immunol. 2022. PMID: 35572540 Free PMC article. Review.
-
Role of T Regulatory Cells and Myeloid-Derived Suppressor Cells in COVID-19.J Immunol Res. 2022 Apr 19;2022:5545319. doi: 10.1155/2022/5545319. eCollection 2022. J Immunol Res. 2022. PMID: 35497875 Free PMC article. Review.
-
Role of myeloid-derived suppressor cells in viral respiratory infections; Hints for discovering therapeutic targets for COVID-19.Biomed Pharmacother. 2021 Dec;144:112346. doi: 10.1016/j.biopha.2021.112346. Epub 2021 Oct 15. Biomed Pharmacother. 2021. PMID: 34678727 Free PMC article. Review.
References
-
- Schreiber RD, Old LJ, Smyth MJ, Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion, Science 331 (2011) 1565–1570. - PubMed
-
- Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De BP, Van Ginderachter JA, Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity, Blood 111 (2008) 4233–4244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

