Photoimmunotherapy of Ovarian Cancer: A Unique Niche in the Management of Advanced Disease
- PMID: 31783651
- PMCID: PMC6966499
- DOI: 10.3390/cancers11121887
Photoimmunotherapy of Ovarian Cancer: A Unique Niche in the Management of Advanced Disease
Abstract
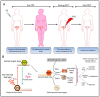
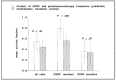
Ovarian cancer (OvCa) is the leading cause of gynecological cancer-related deaths in the United States, with five-year survival rates of 15-20% for stage III cancers and 5% for stage IV cancers. The standard of care for advanced OvCa involves surgical debulking of disseminated disease in the peritoneum followed by chemotherapy. Despite advances in treatment efficacy, the prognosis for advanced stage OvCa patients remains poor and the emergence of chemoresistant disease localized to the peritoneum is the primary cause of death. Therefore, a complementary modality that is agnostic to typical chemo- and radio-resistance mechanisms is urgently needed. Photodynamic therapy (PDT), a photochemistry-based process, is an ideal complement to standard treatments for residual disease. The confinement of the disease in the peritoneal cavity makes it amenable for regionally localized treatment with PDT. PDT involves photochemical generation of cytotoxic reactive molecular species (RMS) by non-toxic photosensitizers (PSs) following exposure to non-harmful visible light, leading to localized cell death. However, due to the complex topology of sensitive organs in the peritoneum, diffuse intra-abdominal PDT induces dose-limiting toxicities due to non-selective accumulation of PSs in both healthy and diseased tissue. In an effort to achieve selective damage to tumorous nodules, targeted PS formulations have shown promise to make PDT a feasible treatment modality in this setting. This targeted strategy involves chemical conjugation of PSs to antibodies, referred to as photoimmunoconjugates (PICs), to target OvCa specific molecular markers leading to enhanced therapeutic outcomes while reducing off-target toxicity. In light of promising results of pilot clinical studies and recent preclinical advances, this review provides the rationale and methodologies for PIC-based PDT, or photo-immunotherapy (PIT), in the context of OvCa management.
Keywords: EGFR; Ovarian cancer; photodynamic therapy; photoimmunoconjugates; photoimmunotherapy; targeted therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

