Decrease of miR-19b-3p in Brain Microvascular Endothelial Cells Attenuates Meningitic Escherichia coli-Induced Neuroinflammation via TNFAIP3-Mediated NF-κB Inhibition
- PMID: 31783671
- PMCID: PMC6963872
- DOI: 10.3390/pathogens8040268
Decrease of miR-19b-3p in Brain Microvascular Endothelial Cells Attenuates Meningitic Escherichia coli-Induced Neuroinflammation via TNFAIP3-Mediated NF-κB Inhibition
Abstract
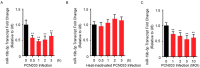
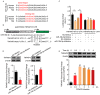
Meningitic Escherichia coli can traverse the host's blood-brain barrier (BBB) and induce severe neuroinflammatory damage to the central nervous system (CNS). During this process, the host needs to reasonably balance the battle between bacteria and brain microvascular endothelial cells (BMECs) to minimize inflammatory damage, but this quenching of neuroinflammatory responses at the BBB is unclear. MicroRNAs (miRNAs) are widely recognized as key negative regulators in many pathophysiological processes, including inflammatory responses. Our previous transcriptome sequencing revealed numbers of differential miRNAs in BMECs upon meningitic E. coli infection; we next sought to explore whether and how these miRNAs worked to modulate neuroinflammatory responses at meningitic E. coli entry of the BBB. Here, we demonstrated in vivo and in vitro that meningitic E. coli infection of BMECs significantly downregulated miR-19b-3p, which led to attenuated production of proinflammatory cytokines and chemokines via increasing the expression of TNFAIP3, a negative regulator of NF-κB signaling. Moreover, in vivo injection of miR-19b-3p mimics during meningitic E. coli challenge further aggravated the inflammatory damage to mice brains. These in vivo and in vitro findings indicate a novel quenching mechanism of the host by attenuating miR-19b-3p/TNFAIP3/NF-κB signaling in BMECs in response to meningitic E. coli, thus preventing CNS from further neuroinflammatory damage.
Keywords: NF-κB; TNFAIP3; brain microvascular endothelial cells; meningitic Escherichia coli; miR-19b-3p; neuroinflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

