Development of clinically relevant in vivo metastasis models using human bone discs and breast cancer patient-derived xenografts
- PMID: 31783893
- PMCID: PMC6884811
- DOI: 10.1186/s13058-019-1220-2
Development of clinically relevant in vivo metastasis models using human bone discs and breast cancer patient-derived xenografts
Abstract
Background: Late-stage breast cancer preferentially metastasises to bone; despite advances in targeted therapies, this condition remains incurable. The lack of clinically relevant models for studying breast cancer metastasis to a human bone microenvironment has stunted the development of effective treatments for this condition. To address this problem, we have developed humanised mouse models in which breast cancer patient-derived xenografts (PDXs) metastasise to human bone implants with low variability and high frequency.
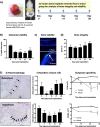
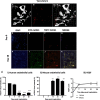
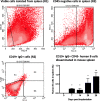
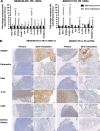
Methods: To model the human bone environment, bone discs from femoral heads of patients undergoing hip replacement surgery were implanted subcutaneously into NOD/SCID mice. For metastasis studies, 7 patient-derived xenograft tumours (PDX: BB3RC32, ER+ PR+ HER2-; BB2RC08, ER+ PR+ ER2-; BB6RC37, ER- PR- HER2- and BB6RC39, ER+ PR+ HER2+), MDA-MB-231-luc2, T47D-luc2 or MCF7-Luc2 cells were injected into the 4th mammary ducts and metastases monitored by luciferase imaging and confirmed on histological sections. Bone integrity, viability and vascularisation were assessed by uCT, calcein uptake and histomorphometry. Expression profiling of genes/proteins during different stages of metastasis were assessed by whole genome Affymetrix array, real-time PCR and immunohistochemistry. Importance of IL-1 was confirmed following anakinra treatment.
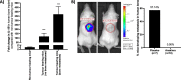
Results: Implantation of femoral bone provided a metabolically active, human-specific site for tumour cells to metastasise to. After 4 weeks, bone implants were re-vascularised and demonstrated active bone remodelling (as evidenced by the presence of osteoclasts, osteoblasts and calcein uptake). Restricting bone implants to the use of subchondral bone and introduction of cancer cells via intraductal injection maximised metastasis to human bone implants. MDA-MB-231 cells specifically metastasised to human bone (70% metastases) whereas T47D, MCF7, BB3RC32, BB2RC08, and BB6RC37 cells metastasised to both human bone and mouse bones. Importantly, human bone was the preferred metastatic site especially from ER+ PDX (100% metastasis human bone compared with 20-75% to mouse bone), whereas ER-ve PDX developed metastases in 20% of human and 20% of mouse bone. Breast cancer cells underwent a series of molecular changes as they progressed from primary tumours to bone metastasis including altered expression of IL-1B, IL-1R1, S100A4, CTSK, SPP1 and RANK. Inhibiting IL-1B signalling significantly reduced bone metastasis.
Conclusions: Our reliable and clinically relevant humanised mouse models provide significant advancements in modelling of breast cancer bone metastasis.
Keywords: Bone metastasis; Breast cancer; ER+; ER−; PDX.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Human breast cancer bone metastasis in vitro and in vivo: a novel 3D model system for studies of tumour cell-bone cell interactions.Clin Exp Metastasis. 2015 Oct;32(7):689-702. doi: 10.1007/s10585-015-9737-y. Epub 2015 Aug 1. Clin Exp Metastasis. 2015. PMID: 26231669
-
IL-1 drives breast cancer growth and bone metastasis in vivo.Oncotarget. 2016 Nov 15;7(46):75571-75584. doi: 10.18632/oncotarget.12289. Oncotarget. 2016. PMID: 27765923 Free PMC article.
-
Oestrogen receptor positive breast cancer metastasis to bone: inhibition by targeting the bone microenvironment in vivo.Clin Exp Metastasis. 2016 Mar;33(3):211-24. doi: 10.1007/s10585-015-9770-x. Epub 2015 Nov 19. Clin Exp Metastasis. 2016. PMID: 26585891
-
Parathyroid hormone-related protein and bone metastases.Cancer. 1997 Oct 15;80(8 Suppl):1572-80. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1572::aid-cncr7>3.3.co;2-d. Cancer. 1997. PMID: 9362424 Review.
-
Animal models of bone metastasis.Cancer. 2003 Feb 1;97(3 Suppl):748-57. doi: 10.1002/cncr.11150. Cancer. 2003. PMID: 12548572 Review.
Cited by
-
The In Vivo Selection Method in Breast Cancer Metastasis.Int J Mol Sci. 2021 Feb 14;22(4):1886. doi: 10.3390/ijms22041886. Int J Mol Sci. 2021. PMID: 33672831 Free PMC article. Review.
-
Parathyroid hormone 1 receptor signaling mediates breast cancer metastasis to bone in mice.JCI Insight. 2023 Mar 8;8(5):e157390. doi: 10.1172/jci.insight.157390. JCI Insight. 2023. PMID: 36692956 Free PMC article.
-
Interleukins as Mediators of the Tumor Cell-Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis.Int J Mol Sci. 2021 Mar 12;22(6):2898. doi: 10.3390/ijms22062898. Int J Mol Sci. 2021. PMID: 33809315 Free PMC article. Review.
-
Cathepsin K regulates the tumor growth and metastasis by IL-17/CTSK/EMT axis and mediates M2 macrophage polarization in castration-resistant prostate cancer.Cell Death Dis. 2022 Sep 22;13(9):813. doi: 10.1038/s41419-022-05215-8. Cell Death Dis. 2022. PMID: 36138018 Free PMC article.
-
TRPS1 regulates the opposite effect of progesterone via RANKL in endometrial carcinoma and breast carcinoma.Cell Death Discov. 2023 Jun 21;9(1):185. doi: 10.1038/s41420-023-01484-0. Cell Death Discov. 2023. PMID: 37344459 Free PMC article.
References
-
- Tulotta C, Groenewoud A, Snaar-Jagalska BE, Ottewell P. Animal models of breast cancer bone metastasis. Methods Mol Biol. 1914;2019:309–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

