Oral vitamin A supplementation of porcine epidemic diarrhea virus infected gilts enhances IgA and lactogenic immune protection of nursing piglets
- PMID: 31783923
- PMCID: PMC6884901
- DOI: 10.1186/s13567-019-0719-y
Oral vitamin A supplementation of porcine epidemic diarrhea virus infected gilts enhances IgA and lactogenic immune protection of nursing piglets
Abstract
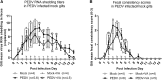
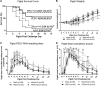
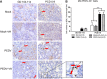
Vitamin A (VA) has pleiotropic effects on the immune system and is critical for mucosal immune function and intestinal lymphocyte trafficking. We hypothesized that oral VA supplementation of porcine epidemic diarrhea virus (PEDV)-infected pregnant gilts would enhance the gut-mammary gland-secretory IgA axis to boost lactogenic immunity and passive protection of nursing piglets against PEDV challenge. Gilts received daily oral retinyl acetate (30 000 IU) starting at gestation day 76 throughout lactation. At 3-4 weeks pre-partum, VA-supplemented (PEDV + VA) and non-supplemented (PEDV) gilts were PEDV or mock inoculated (mock + VA and mock, respectively). PEDV + VA gilts had decreased mean PEDV RNA shedding titers and diarrhea scores. To determine if lactogenic immunity correlated with protection, all piglets were PEDV-challenged at 3-5 days post-partum. The survival rate of PEDV + VA litters was 74.2% compared with 55.9% in PEDV litters. Mock and mock + VA litter survival rates were 5.7% and 8.3%, respectively. PEDV + VA gilts had increased PEDV IgA antibody secreting cells and PEDV IgA antibodies in serum pre-partum and IgA+β7+ (gut homing) cells in milk post piglet challenge compared with PEDV gilts. Our findings suggest that oral VA supplementation may act as an adjuvant during pregnancy, enhancing maternal IgA and lactogenic immune protection in nursing piglets.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Stage of Gestation at Porcine Epidemic Diarrhea Virus Infection of Pregnant Swine Impacts Maternal Immunity and Lactogenic Immune Protection of Neonatal Suckling Piglets.Front Immunol. 2019 Apr 24;10:727. doi: 10.3389/fimmu.2019.00727. eCollection 2019. Front Immunol. 2019. PMID: 31068924 Free PMC article.
-
Maternal immunization and vitamin A sufficiency impact sow primary adaptive immunity and passive protection to nursing piglets against porcine epidemic diarrhea virus infection.Front Immunol. 2024 May 15;15:1397118. doi: 10.3389/fimmu.2024.1397118. eCollection 2024. Front Immunol. 2024. PMID: 38812505 Free PMC article.
-
Isolation and oral immunogenicity assessment of porcine epidemic diarrhea virus NH-TA2020 strain: One of the predominant strains circulating in China from 2017 to 2021.Virol Sin. 2022 Oct;37(5):646-655. doi: 10.1016/j.virs.2022.08.002. Epub 2022 Aug 9. Virol Sin. 2022. PMID: 35961502 Free PMC article.
-
Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts.Virus Res. 2016 Dec 2;226:93-107. doi: 10.1016/j.virusres.2016.05.016. Epub 2016 May 19. Virus Res. 2016. PMID: 27212686 Free PMC article. Review.
-
Host Factors Affecting Generation of Immunity Against Porcine Epidemic Diarrhea Virus in Pregnant and Lactating Swine and Passive Protection of Neonates.Pathogens. 2020 Feb 18;9(2):130. doi: 10.3390/pathogens9020130. Pathogens. 2020. PMID: 32085410 Free PMC article. Review.
Cited by
-
Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control.Virus Res. 2020 Sep;286:198045. doi: 10.1016/j.virusres.2020.198045. Epub 2020 Jun 2. Virus Res. 2020. PMID: 32502552 Free PMC article. Review.
-
Impact of maternal nutrition in viral infections during pregnancy.Biochim Biophys Acta Mol Basis Dis. 2021 Nov 1;1867(11):166231. doi: 10.1016/j.bbadis.2021.166231. Epub 2021 Jul 31. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 34343638 Free PMC article. Review.
-
Programming Effects of Maternal Nutrition on Intestinal Development and Microorganisms of Offspring: A Review on Pigs.Microorganisms. 2025 May 17;13(5):1151. doi: 10.3390/microorganisms13051151. Microorganisms. 2025. PMID: 40431323 Free PMC article. Review.
-
Vitamin A Requirements in Pregnancy and Lactation.Curr Dev Nutr. 2020 Aug 24;4(10):nzaa142. doi: 10.1093/cdn/nzaa142. eCollection 2020 Oct. Curr Dev Nutr. 2020. PMID: 32999954 Free PMC article. Review.
-
The Role of Nutrition in COVID-19 Susceptibility and Severity of Disease: A Systematic Review.J Nutr. 2021 Jul 1;151(7):1854-1878. doi: 10.1093/jn/nxab059. J Nutr. 2021. PMID: 33982105 Free PMC article.
References
-
- Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Döpfer D, Fazil A, Fischer-Walker CL, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12:e1001921. doi: 10.1371/journal.pmed.1001921. - DOI - PMC - PubMed
-
- Saif LJ, Pensaert MB, Sestak K, Yeo SG, Jung K. Coronaviruses. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Diseases of swine. Hoboken: Wiley-Blackwell; 2012.
-
- Paarlberg PL (2014) Updated estimated economic welfare impacts of porcine epidemic diarrhea virus (PEDv). In: Working Paper #14-4. Purdue University
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

