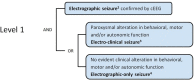
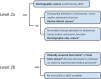
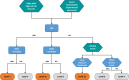
Neonatal seizures: Case definition & guidelines for data collection, analysis, and presentation of immunization safety data
- PMID: 31783981
- PMCID: PMC6899436
- DOI: 10.1016/j.vaccine.2019.05.031
Neonatal seizures: Case definition & guidelines for data collection, analysis, and presentation of immunization safety data
Keywords: Adverse event; Case definition; Guidelines; Immunization; Neonatal seizures.
Conflict of interest statement
The authors declared that there is no conflict of interest.
Similar articles
-
Neurodevelopmental delay: Case definition & guidelines for data collection, analysis, and presentation of immunization safety data.Vaccine. 2019 Dec 10;37(52):7623-7641. doi: 10.1016/j.vaccine.2019.05.027. Vaccine. 2019. PMID: 31783983 Free PMC article. Review. No abstract available.
-
Acute wheeze in the pediatric population: Case definition & guidelines for data collection, analysis, and presentation of immunization safety data.Vaccine. 2019 Jan 7;37(2):392-399. doi: 10.1016/j.vaccine.2017.01.083. Epub 2017 May 5. Vaccine. 2019. PMID: 28483201 No abstract available.
-
Acute aseptic arthritis: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data.Vaccine. 2019 Jan 7;37(2):384-391. doi: 10.1016/j.vaccine.2017.08.087. Epub 2018 Oct 17. Vaccine. 2019. PMID: 30342899 Review. No abstract available.
-
Postpartum endometritis and infection following incomplete or complete abortion: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data.Vaccine. 2019 Dec 10;37(52):7585-7595. doi: 10.1016/j.vaccine.2019.09.101. Vaccine. 2019. PMID: 31783980 Free PMC article. Review. No abstract available.
-
Chorioamnionitis: Case definition & guidelines for data collection, analysis, and presentation of immunization safety data.Vaccine. 2019 Dec 10;37(52):7610-7622. doi: 10.1016/j.vaccine.2019.05.030. Vaccine. 2019. PMID: 31783982 Free PMC article. Review. No abstract available.
Cited by
-
Temporal evolution of electrographic seizures in newborn infants with hypoxic-ischaemic encephalopathy requiring therapeutic hypothermia: a secondary analysis of the ANSeR studies.Lancet Child Adolesc Health. 2024 Mar;8(3):214-224. doi: 10.1016/S2352-4642(23)00296-1. Epub 2024 Jan 18. Lancet Child Adolesc Health. 2024. PMID: 38246187 Free PMC article. Clinical Trial.
-
Global Perspectives on Immunization During Pregnancy and Priorities for Future Research and Development: An International Consensus Statement.Front Immunol. 2020 Jun 24;11:1282. doi: 10.3389/fimmu.2020.01282. eCollection 2020. Front Immunol. 2020. PMID: 32670282 Free PMC article. Review.
-
Incidence and predictors of neonatal seizures among neonates admitted in Debre Markos Comprehensive Specialized Hospital, Northwest Ethiopia. A prospective follow-up study.Heliyon. 2024 Apr 22;10(9):e29999. doi: 10.1016/j.heliyon.2024.e29999. eCollection 2024 May 15. Heliyon. 2024. PMID: 38707374 Free PMC article.
-
The Role of Amplitude-Integrated Electroencephalography (aEEG) in Monitoring Infants with Neonatal Seizures and Predicting Their Neurodevelopmental Outcome.Children (Basel). 2023 May 3;10(5):833. doi: 10.3390/children10050833. Children (Basel). 2023. PMID: 37238381 Free PMC article.
-
Defining neonatal status epilepticus: A scoping review from the ILAE neonatal task force.Epilepsia Open. 2025 Feb;10(1):40-54. doi: 10.1002/epi4.13090. Epub 2024 Nov 14. Epilepsia Open. 2025. PMID: 39540265 Free PMC article.
References
-
- Barlow W.E., Davis R.L., Glasser J.W., Rhodes P.H., Thompson R.S., Mullooly J.P. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med. 2001;345:656–661. - PubMed
-
- Bonhoeffer J., Menkes J., Gold M.S., de Souza-Brito G., Fisher M.C., Halsey N. Generalized convulsive seizure as an adverse event following immunization: case definition and guidelines for data collection, analysis, and presentation. Vaccine. 2004;22:557–562. - PubMed
-
- Plouin P., Kaminska A. Chapter 51 - neonatal seizures. In: Dulac O., Lassonde M., Sarnat H.B., editors. Handbook of clinical neurology. Elsevier; 2013. pp. 467–476. - PubMed
-
- Vasudevan C., Levene M. Epidemiology and aetiology of neonatal seizures. Sem Fetal Neonatal Med. 2013;18:185–191. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical