A collagen-binding protein enables molecular imaging of kidney fibrosis in vivo
- PMID: 31784048
- PMCID: PMC7115881
- DOI: 10.1016/j.kint.2019.08.029
A collagen-binding protein enables molecular imaging of kidney fibrosis in vivo
Abstract
Pathological deposition of collagen is a hallmark of kidney fibrosis. To illustrate this process we employed multimodal optical imaging to visualize and quantify collagen deposition in murine models of kidney fibrosis (ischemia-reperfusion or unilateral ureteral obstruction) using the collagen-binding adhesion protein CNA35. For in vivo imaging, we used hybrid computed tomography-fluorescence molecular tomography and CNA35 labeled with the near-infrared fluorophore Cy7. Upon intravenous injection, CNA35-Cy7 accumulation was significantly higher in fibrotic compared to non-fibrotic kidneys. This difference was not detected for a non-specific scrambled version of CNA35-Cy7. Ex vivo, on kidney sections of mice and patients with renal fibrosis, CNA35-FITC co-localized with fibrotic collagen type I and III, but not with the basement membrane collagen type IV. Following intravenous injection, CNA35-FITC bound to both interstitial and perivascular fibrotic areas. In line with this perivascular accumulation, we observed significant perivascular fibrosis in the mouse models and in biopsy sections from patients with chronic kidney disease using computer-based morphometry quantification. Thus, molecular imaging of collagen using CNA35 enabled specific non-invasive quantification of kidney fibrosis. Collagen imaging revealed significant perivascular fibrosis as a consistent component next to the more commonly assessed interstitial fibrosis. Our results lay the basis for further probe and protocol optimization towards the clinical translation of molecular imaging of kidney fibrosis.
Keywords: chronic kidney disease (CKD); collagen; extracellular matrix; molecular imaging; non-invasive imaging; renal fibrosis.
Copyright © 2019 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
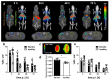
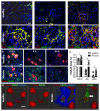

Comment in
-
The scar that never felt a wound.Kidney Int. 2020 Mar;97(3):460-462. doi: 10.1016/j.kint.2019.10.006. Kidney Int. 2020. PMID: 32087889
References
-
- Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med. 2019;65:16–36. - PubMed
-
- Klinkhammer BM, Goldschmeding R, Floege J, et al. Treatment of Renal Fibrosis-Turning Challenges into Opportunities. Adv Chronic Kidney Dis. 2017;24:117–129. - PubMed
-
- Krahn KN, Bouten CV, van Tuijl S, et al. Fluorescently labeled collagen binding proteins allow specific visualization of collagen in tissues and live cell culture. Anal Biochem. 2006;350:177–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

