Tau-positron emission tomography correlates with neuropathology findings
- PMID: 31784374
- PMCID: PMC7067654
- DOI: 10.1016/j.jalz.2019.09.079
Tau-positron emission tomography correlates with neuropathology findings
Abstract
Introduction: Comparison of tau (flortaucipir) positron emission tomography (FTP-PET) to autopsy is important to demonstrate the relationship of FTP-PET to neuropathologic findings.
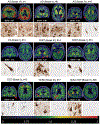
Methods: Autopsies were performed on 26 participants who had antemortem FTP-PET. FTP-PET standardized uptake value ratios (SUVRs) were compared to autopsy diagnoses and Braak tangle stage. Quantitative tau burden was compared to regional FTP-PET signal.
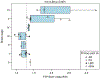
Results: Participants with Braak stages of IV or greater had elevated FTP-PET signal. FTP-PET was elevated in participants with Alzheimer's disease. An FTP-PET SUVR cut point of 1.29 was determined to be optimal. Quantitative measurements of hippocampal and temporal lobe tau burden were highly correlated to FTP-PET signal (rho's from 0.61 to 0.70, P ≤ .02).
Discussion: Elevated FTP-PET reflects Braak IV or greater neuropathology. Participants with primary age-related tauopathy and hippocampal sclerosis did not show elevated FTP-PET signal. Secondary neuropathologic diagnoses of Alzheimer's disease neuropathologic change can lead to borderline elevated FTP-PET signal.
Keywords: Alzheimer's disease; Autopsy; Braak tangle stage; PET; Tau; flortaucipir.
© 2019 the Alzheimer's Association.
Figures


References
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. - PubMed
-
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61:378–84. - PubMed
-
- Duyckaerts C, Bennecib M, Grignon Y, Uchihara T, He Y, Piette F, et al. Modeling the relation between neurofibrillary tangles and intellectual status. Neurobiol Aging. 1997;18:267–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG041851/GF/NIH HHS/United States
- R01 AG041851/AG/NIA NIH HHS/United States
- R01 AG034676/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- R01 AG054449/AG/NIA NIH HHS/United States
- R01 NS089544/GF/NIH HHS/United States
- R01 AG011378/AG/NIA NIH HHS/United States
- R21 NS094489/GF/NIH HHS/United States
- RF1 AG057547/AG/NIA NIH HHS/United States
- R01 NS097495/GF/NIH HHS/United States
- Mayo Foundation for Medical Education and Research/International
- R37 AG011378/AG/NIA NIH HHS/United States
- R01 NS089544/NS/NINDS NIH HHS/United States
- U01 NS100620/NS/NINDS NIH HHS/United States
- Robert Wood Johnson Foundation/International
- R21 NS094489/NS/NINDS NIH HHS/United States
- R01 NS089757/NS/NINDS NIH HHS/United States
- R01 DC010367/DC/NIDCD NIH HHS/United States
- R01 NS097495/NS/NINDS NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
- U01 AG006786/AG/NIA NIH HHS/United States
- U54 NS100693/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

