GPR108 Is a Highly Conserved AAV Entry Factor
- PMID: 31784416
- PMCID: PMC7000996
- DOI: 10.1016/j.ymthe.2019.11.005
GPR108 Is a Highly Conserved AAV Entry Factor
Abstract
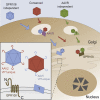
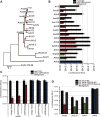
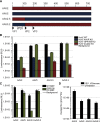
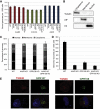
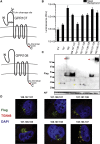
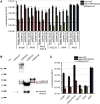
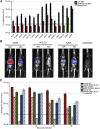
Adeno-associated virus (AAV) is a highly promising gene transfer vector, yet major cellular requirements for AAV entry are poorly understood. Using a genome-wide CRISPR screen for entry of evolutionarily divergent serotype AAVrh32.33, we identified GPR108, a member of the G protein-coupled receptor superfamily, as an AAV entry factor. Of greater than 20 divergent AAVs across all AAV clades tested in human cell lines, only AAV5 transduction was unaffected in the GPR108 knockout (KO). GPR108 dependency was further shown in murine and primary cells in vitro. These findings are further validated in vivo, as the Gpr108 KO mouse demonstrates 10- to 100-fold reduced expression for AAV8 and rh32.33 but not AAV5. Mechanistically, both GPR108 N- and C-terminal domains are required for transduction, and on the capsid, a VP1 unique domain that is not conserved on AAV5 can be transferred to confer GPR108 independence onto AAV2 chimeras. In vitro binding and fractionation studies indicate reduced nuclear import and cytosolic accumulation in the absence of GPR108. We thus have identified the second of two AAV entry factors that is conserved between mice and humans relevant both in vitro and in vivo, further providing a mechanistic understanding to the tropism of AAV gene therapy vectors.
Keywords: AAV; CRISPR screen; GPR108; adeno-associated virus; endosomal escape; entry; in vivo; receptor.
Copyright © 2019 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
AAV Entry: Filling in the Blanks.Mol Ther. 2020 Feb 5;28(2):346-347. doi: 10.1016/j.ymthe.2020.01.015. Epub 2020 Jan 28. Mol Ther. 2020. PMID: 31991107 Free PMC article. No abstract available.
Similar articles
-
Identification of SLC35A1 as an essential host factor for the transduction of multi-serotype recombinant adeno-associated virus (AAV) vectors.mBio. 2025 Jan 8;16(1):e0326824. doi: 10.1128/mbio.03268-24. Epub 2024 Nov 27. mBio. 2025. PMID: 39601564 Free PMC article.
-
Chimeric Capsid Proteins Impact Transduction Efficiency of Haploid Adeno-Associated Virus Vectors.Viruses. 2019 Dec 9;11(12):1138. doi: 10.3390/v11121138. Viruses. 2019. PMID: 31835440 Free PMC article.
-
The Golgi Calcium ATPase Pump Plays an Essential Role in Adeno-associated Virus Trafficking and Transduction.J Virol. 2020 Oct 14;94(21):e01604-20. doi: 10.1128/JVI.01604-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32817219 Free PMC article.
-
New recombinant serotypes of AAV vectors.Curr Gene Ther. 2005 Jun;5(3):285-97. doi: 10.2174/1566523054065057. Curr Gene Ther. 2005. PMID: 15975006 Review.
-
Expressing Transgenes That Exceed the Packaging Capacity of Adeno-Associated Virus Capsids.Hum Gene Ther Methods. 2016 Feb;27(1):1-12. doi: 10.1089/hgtb.2015.140. Hum Gene Ther Methods. 2016. PMID: 26757051 Free PMC article. Review.
Cited by
-
Structural and antigenic characterization of the avian adeno-associated virus capsid.J Virol. 2023 Oct 31;97(10):e0078023. doi: 10.1128/jvi.00780-23. Epub 2023 Sep 13. J Virol. 2023. PMID: 37702486 Free PMC article.
-
Hydroxylation of N-acetylneuraminic Acid Influences the in vivo Tropism of N-linked Sialic Acid-Binding Adeno-Associated Viruses AAV1, AAV5, and AAV6.Front Med (Lausanne). 2021 Dec 21;8:732095. doi: 10.3389/fmed.2021.732095. eCollection 2021. Front Med (Lausanne). 2021. PMID: 35036407 Free PMC article.
-
Pooled Screens Identify GPR108 and TM9SF2 as Host Cell Factors Critical for AAV Transduction.Mol Ther Methods Clin Dev. 2020 Mar 17;17:601-611. doi: 10.1016/j.omtm.2020.03.012. eCollection 2020 Jun 12. Mol Ther Methods Clin Dev. 2020. PMID: 32280726 Free PMC article.
-
AAV11 enables efficient retrograde targeting of projection neurons and enhances astrocyte-directed transduction.Nat Commun. 2023 Jun 26;14(1):3792. doi: 10.1038/s41467-023-39554-7. Nat Commun. 2023. PMID: 37365155 Free PMC article.
-
Progress in AAV-Mediated In Vivo Gene Therapy and Its Applications in Central Nervous System Diseases.Int J Mol Sci. 2025 Feb 28;26(5):2213. doi: 10.3390/ijms26052213. Int J Mol Sci. 2025. PMID: 40076831 Free PMC article. Review.
References
-
- Russell S., Bennett J., Wellman J.A., Chung D.C., Yu Z.F., Tillman A., Wittes J., Pappas J., Elci O., McCague S. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. - PMC - PubMed
-
- Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. - PubMed
-
- Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. - PubMed
-
- Voretigene neparvovec-rzyl (Luxturna) for inherited retinal dystrophy. Med. Lett. Drugs Ther. 2018;60:53–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

