Pharmacological and behavioural interventions to promote smoking cessation in adults with schizophrenia and bipolar disorders: a systematic review and meta-analysis of randomised trials
- PMID: 31784428
- PMCID: PMC6924825
- DOI: 10.1136/bmjopen-2018-027389
Pharmacological and behavioural interventions to promote smoking cessation in adults with schizophrenia and bipolar disorders: a systematic review and meta-analysis of randomised trials
Abstract
Objective: Smoking in people with serious mental illness is a major public health problem and contributes to significant levels of morbidity and mortality. The aim of the review was to systematically examine the efficacy of methods used to aid smoking cessation in people with serious mental illness.
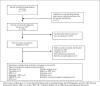
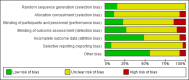
Method: A systematic review and meta-analysis of randomised controlled trials to compare the effectiveness and safety of pharmacological and behavioural programmes for smoking cessation in people with serious mental illness. Electronic databases were searched for trials to July 2018. We used the Cochrane Collaboration's tool for assessing the risk of bias.
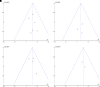
Results: Twenty-eight randomised controlled trials were identified. Varenicline increased the likelihood of smoking cessation at both 3 months (risk ratio (RR) 3.56, 95% CI 1.82 to 6.96, p=0.0002) and at 6 months (RR 3.69, 95% CI 1.08 to 12.60, p=0.04). Bupropion was effective at 3 months (RR 3.96, 95% CI 1.86 to 8.40, p=0.0003), especially at a dose of 300 mg/day, but there was no evidence of effect at 6 months (RR 2.22, 95% CI 0.52 to 9.47, p=0.28). In one small study, nicotine therapy proved effective at increasing smoking cessation up to a period of 3 months. Bupropion used in conjunction with nicotine replacement therapy showed more effect than single use. Behavioural and bespoke interventions showed little overall benefit. Side effects were found to be low.
Conclusion: The new information of this review was the effectiveness of varenicline for smoking cessation at both 3 and 6 months and the lack of evidence to support the use of both bupropion and nicotine products for sustained abstinence longer than 3 months. Overall, the review found relatively few studies in this population.
Keywords: bupropion; nicotine replacement; serious mental illness; smoking cessation; varenicline.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RP and DJS declared no competing interests. JG has received research funding from MRC, ESRC, NIHR, Stanley Medical Research Institute and has received donations of drugs supplies for trials from Sanofi-Aventis and GSK. He has acted as an expert witness for Dr Reddys.
Figures






Similar articles
-
Smoking cessation for people with severe mental illness (SCIMITAR+): a pragmatic randomised controlled trial.Lancet Psychiatry. 2019 May;6(5):379-390. doi: 10.1016/S2215-0366(19)30047-1. Epub 2019 Apr 8. Lancet Psychiatry. 2019. PMID: 30975539 Free PMC article. Clinical Trial.
-
Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation.Cochrane Database Syst Rev. 2019 Apr 18;4(4):CD013308. doi: 10.1002/14651858.CD013308. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2023 Jun 19;6:CD013308. doi: 10.1002/14651858.CD013308.pub2. PMID: 30997928 Free PMC article. Updated.
-
Interventions for Tobacco Cessation in Adults, Including Pregnant Persons: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force.JAMA. 2021 Jan 19;325(3):280-298. doi: 10.1001/jama.2020.23541. JAMA. 2021. PMID: 33464342
-
Combination therapy of varenicline and bupropion in smoking cessation: A meta-analysis of the randomized controlled trials.Compr Psychiatry. 2019 Nov;95:152125. doi: 10.1016/j.comppsych.2019.152125. Epub 2019 Sep 5. Compr Psychiatry. 2019. PMID: 31669972
-
Primary Care-Relevant Interventions for Tobacco and Nicotine Use Prevention and Cessation in Children and Adolescents: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force.JAMA. 2020 Apr 28;323(16):1599-1608. doi: 10.1001/jama.2020.3332. JAMA. 2020. PMID: 32343335
Cited by
-
Integrated Smoking Cessation for Smokers With Serious Mental Illness: Protocol for a Convergent Mixed Methods Implementation Evaluation Study.JMIR Res Protoc. 2021 Jul 27;10(7):e25390. doi: 10.2196/25390. JMIR Res Protoc. 2021. PMID: 34313603 Free PMC article.
-
Identification and management of cardiometabolic risk in subjects with schizophrenia spectrum disorders: A Delphi expert consensus study.Eur Psychiatry. 2021 Jan 8;64(1):e7. doi: 10.1192/j.eurpsy.2020.115. Eur Psychiatry. 2021. PMID: 33413701 Free PMC article.
-
The detrimental effects of smoking on the course and outcome in adults with bipolar disorder-A narrative review.Front Psychiatry. 2023 Jan 9;13:1114432. doi: 10.3389/fpsyt.2022.1114432. eCollection 2022. Front Psychiatry. 2023. PMID: 36699491 Free PMC article. Review.
-
Inequity in smoking cessation clinical trials testing pharmacotherapies: exclusion of smokers with mental health disorders.Tob Control. 2023 Jul;32(4):489-496. doi: 10.1136/tobaccocontrol-2021-056843. Epub 2021 Dec 3. Tob Control. 2023. PMID: 34862325 Free PMC article. Review.
-
Case report: Schizophrenia and hypertrophic osteoarthropathy, a rare syndrome hiding a life-threatening condition.Clin Case Rep. 2022 Jan 12;10(1):e05247. doi: 10.1002/ccr3.5247. eCollection 2022 Jan. Clin Case Rep. 2022. PMID: 35059198 Free PMC article.
References
-
- Scottish Health Survey 2. The Scottish Government, Edinburgh, 2010. www. scotland.gov.uk/publications.
