Protocol for a double-blind, randomised, placebo-controlled pilot study for assessing the feasibility and efficacy of faecal microbiota transplant in a paediatric Crohn's disease population: PediCRaFT Trial
- PMID: 31784432
- PMCID: PMC6924772
- DOI: 10.1136/bmjopen-2019-030120
Protocol for a double-blind, randomised, placebo-controlled pilot study for assessing the feasibility and efficacy of faecal microbiota transplant in a paediatric Crohn's disease population: PediCRaFT Trial
Abstract
Introduction: Crohn's disease (CD) is a chronic inflammatory condition with transmural involvement of the gastrointestinal tract. Extraintestinal manifestations are common, and the disease burden on patients and the healthcare system is significant. While treatment options have expanded in recent years, they have mainly focused on dampening the immune response, thus carrying notable risks associated with long-term immunosuppression. Faecal microbiota transplant (FMT) targets inflammatory bowel disease (IBD) by modifying intestinal dysbiosis. Limited adult and paediatric data have demonstrated a favourable response to FMT in IBD; however, no randomised controlled trial has yet been published in paediatrics. This double-blind, randomised, placebo-controlled pilot study will assess feasibility and efficacy outcomes of FMT in a paediatric CD population.
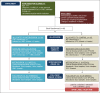
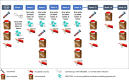
Methods and analysis: Forty-five patients between the ages of 3 and 17 years, with established CD or IBD unclassified, will be enrolled 2:1 to undergo FMT intervention or placebo control. Participants will undergo a colonoscopic infusion to the terminal ileum at baseline, followed by oral capsules two times per week for 6 weeks. Outcomes will be measured throughout the intervention period and 18 weeks of subsequent follow-up. Primary outcomes will assess feasibility, including patient recruitment, sample collection and rates of adverse events. Secondary outcomes will address clinical efficacy, including change in clinical response, change in urine metabolome and change in microbiome.
Ethics and dissemination: Ethics approval from the local hospital research ethics board was obtained at the primary site (McMaster Children's Hospital, Hamilton), with ethics pending at the secondary site (Centre Hospitalier Universitaire-Sainte-Justine, Montréal). RBX7455 and RBX2660 are human donor-sourced, microbiota-based therapeutic formulations. Both RBX7455 and RBX2660 are currently undergoing clinical trials to support potential US Food and Drug Administration approval. Approval to conduct this paediatric clinical trial was obtained from Health Canada's Biologics and Genetic Therapies Directorate. The results of this trial will be published in peer-reviewed journals and will help inform a large, multicentre trial in the future.
Trial registration number: NCT03378167; pre-results.
Keywords: crohn’s disease; faecal microbiota transplant; inflammatory bowel disease; microbiome; oral microbiota capsules; paediatrics.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
