Lar maintains the homeostasis of the hematopoietic organ in Drosophila by regulating insulin signaling in the niche
- PMID: 31784462
- PMCID: PMC6955224
- DOI: 10.1242/dev.178202
Lar maintains the homeostasis of the hematopoietic organ in Drosophila by regulating insulin signaling in the niche
Abstract
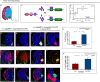
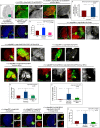
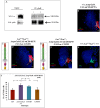
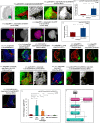
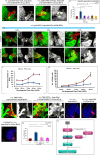
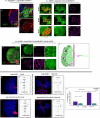
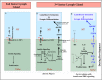
Stem cell compartments in metazoa get regulated by systemic factors as well as local stem cell niche-derived factors. However, the mechanisms by which systemic signals integrate with local factors in maintaining tissue homeostasis remain unclear. Employing the Drosophila lymph gland, which harbors differentiated blood cells, and stem-like progenitor cells and their niche, we demonstrate how a systemic signal interacts and harmonizes with local factor/s to achieve cell type-specific tissue homeostasis. Our genetic analyses uncovered a novel function of Lar, a receptor protein tyrosine phosphatase. Niche-specific loss of Lar leads to upregulated insulin signaling, causing increased niche cell proliferation and ectopic progenitor differentiation. Insulin signaling assayed by PI3K activation is downregulated after the second instar larval stage, a time point that coincides with the appearance of Lar in the hematopoietic niche. We further demonstrate that Lar physically associates with InR and serves as a negative regulator for insulin signaling in the Drosophila larval hematopoietic niche. Whether Lar serves as a localized invariable negative regulator of systemic signals such as insulin in other stem cell niches remains to be explored.
Keywords: Drosophila; Hematopoietic niche; InR-Pi3K-Akt signaling; Lar; Systemic signal.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
Similar articles
-
Drosophila as a Model to Study Cellular Communication Between the Hematopoietic Niche and Blood Progenitors Under Homeostatic Conditions and in Response to an Immune Stress.Front Immunol. 2021 Aug 16;12:719349. doi: 10.3389/fimmu.2021.719349. eCollection 2021. Front Immunol. 2021. PMID: 34484226 Free PMC article. Review.
-
Gene regulatory networks controlling hematopoietic progenitor niche cell production and differentiation in the Drosophila lymph gland.PLoS One. 2012;7(7):e41604. doi: 10.1371/journal.pone.0041604. Epub 2012 Jul 24. PLoS One. 2012. PMID: 22911822 Free PMC article.
-
Bag of Marbles controls the size and organization of the Drosophila hematopoietic niche through interactions with the Insulin-like growth factor pathway and Retinoblastoma-family protein.Development. 2015 Jul 1;142(13):2261-7. doi: 10.1242/dev.121798. Epub 2015 Jun 3. Development. 2015. PMID: 26041767
-
Insulin and Target of rapamycin signaling orchestrate the development of ovarian niche-stem cell units in Drosophila.Development. 2013 Oct;140(20):4145-54. doi: 10.1242/dev.093773. Epub 2013 Sep 11. Development. 2013. PMID: 24026119
-
Regulation of hematopoietic stem cells in the niche.Sci China Life Sci. 2015 Dec;58(12):1209-15. doi: 10.1007/s11427-015-4960-y. Epub 2015 Nov 13. Sci China Life Sci. 2015. PMID: 26563156 Review.
Cited by
-
Regulation of Drosophila Hematopoiesis in Lymph Gland: From a Developmental Signaling Point of View.Int J Mol Sci. 2020 Jul 24;21(15):5246. doi: 10.3390/ijms21155246. Int J Mol Sci. 2020. PMID: 32722007 Free PMC article. Review.
-
Dual role for Headcase in hemocyte progenitor fate determination in Drosophila melanogaster.PLoS Genet. 2024 Oct 28;20(10):e1011448. doi: 10.1371/journal.pgen.1011448. eCollection 2024 Oct. PLoS Genet. 2024. PMID: 39466810 Free PMC article.
-
Systematic analysis of the Frazzled receptor interactome establishes previously unreported regulators of axon guidance.Development. 2023 Aug 1;150(15):dev201636. doi: 10.1242/dev.201636. Epub 2023 Aug 1. Development. 2023. PMID: 37526651 Free PMC article.
-
Drosophila as a Model to Study Cellular Communication Between the Hematopoietic Niche and Blood Progenitors Under Homeostatic Conditions and in Response to an Immune Stress.Front Immunol. 2021 Aug 16;12:719349. doi: 10.3389/fimmu.2021.719349. eCollection 2021. Front Immunol. 2021. PMID: 34484226 Free PMC article. Review.
-
Reinstated Activity of Human Tau-induced Enhanced Insulin Signaling Restricts Disease Pathogenesis by Regulating the Functioning of Kinases/Phosphatases and Tau Hyperphosphorylation in Drosophila.Mol Neurobiol. 2024 Feb;61(2):982-1001. doi: 10.1007/s12035-023-03599-y. Epub 2023 Sep 7. Mol Neurobiol. 2024. PMID: 37674037
References
-
- Ahmad F., Considine R. V. and Goldstein B. J. (1995). Increased abundance of the receptor-type protein-tyrosine phosphatase LAR accounts for the elevated insulin receptor dephosphorylating activity in adipose tissue of obese human subjects. J. Clin. Invest. 95, 2806-2812. 10.1172/JCI117985 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

