Enzyme-mimetic self-catalyzed polymerization of polypeptide helices
- PMID: 31784526
- PMCID: PMC6884638
- DOI: 10.1038/s41467-019-13502-w
Enzyme-mimetic self-catalyzed polymerization of polypeptide helices
Abstract
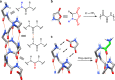
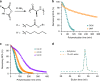
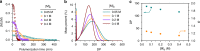
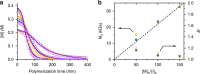
Enzymes provide optimal three-dimensional structures for substrate binding and the subsequent accelerated reaction. Such folding-dependent catalytic behaviors, however, are seldom mechanistically explored with reduced structural complexity. Here, we demonstrate that the α-helix, a much simpler structural motif of enzyme, can facilitate its own growth through the self-catalyzed polymerization of N-carboxyanhydride (NCA) in dichloromethane. The reversible binding between the N terminus of α-helical polypeptides and NCAs promotes rate acceleration of the subsequent ring-opening reaction. A two-stage, Michaelis-Menten-type kinetic model is proposed by considering the binding and reaction between the propagating helical chains and the monomers, and is successfully utilized to predict the molecular weights and molecular-weight distributions of the resulting polymers. This work elucidates the mechanism of helix-induced, enzyme-mimetic catalysis, emphasizes the importance of solvent choice in the discovery of new reaction type, and provides a route for rapid production of well-defined synthetic polypeptides by taking advantage of self-accelerated ring-opening polymerizations.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Purich DL. Enzyme Kinetics: Catalysis and Control. Amsterdam: Elsevier Science; 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

