Alkyladenine DNA glycosylase associates with transcription elongation to coordinate DNA repair with gene expression
- PMID: 31784530
- PMCID: PMC6884549
- DOI: 10.1038/s41467-019-13394-w
Alkyladenine DNA glycosylase associates with transcription elongation to coordinate DNA repair with gene expression
Abstract
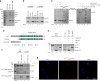
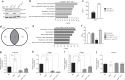
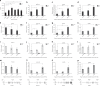
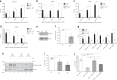
Base excision repair (BER) initiated by alkyladenine DNA glycosylase (AAG) is essential for removal of aberrantly methylated DNA bases. Genome instability and accumulation of aberrant bases accompany multiple diseases, including cancer and neurological disorders. While BER is well studied on naked DNA, it remains unclear how BER efficiently operates on chromatin. Here, we show that AAG binds to chromatin and forms complex with RNA polymerase (pol) II. This occurs through direct interaction with Elongator and results in transcriptional co-regulation. Importantly, at co-regulated genes, aberrantly methylated bases accumulate towards the 3'end in regions enriched for BER enzymes AAG and APE1, Elongator and active RNA pol II. Active transcription and functional Elongator are further crucial to ensure efficient BER, by promoting AAG and APE1 chromatin recruitment. Our findings provide insights into genome stability maintenance in actively transcribing chromatin and reveal roles of aberrantly methylated bases in regulation of gene expression.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Friedberg, E. C. et al. DNA Repair And Mutagenesis (ASM Press, 2006).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

