Calorie restriction slows age-related microbiota changes in an Alzheimer's disease model in female mice
- PMID: 31784610
- PMCID: PMC6884494
- DOI: 10.1038/s41598-019-54187-x
Calorie restriction slows age-related microbiota changes in an Alzheimer's disease model in female mice
Abstract
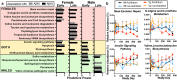
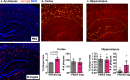
Alzheimer's disease (AD) affects an estimated 5.8 million Americans, and advanced age is the greatest risk factor. AD patients have altered intestinal microbiota. Accordingly, depleting intestinal microbiota in AD animal models reduces amyloid-beta (Aβ) plaque deposition. Age-related changes in the microbiota contribute to immunologic and physiologic decline. Translationally relevant dietary manipulations may be an effective approach to slow microbiota changes during aging. We previously showed that calorie restriction (CR) reduced brain Aβ deposition in the well-established Tg2576 mouse model of AD. Presently, we investigated whether CR alters the microbiome during aging. We found that female Tg2576 mice have more substantial age-related microbiome changes compared to wildtype (WT) mice, including an increase in Bacteroides, which were normalized by CR. Specific gut microbiota changes were linked to Aβ levels, with greater effects in females than in males. In the gut, Tg2576 female mice had an enhanced intestinal inflammatory transcriptional profile, which was reversed by CR. Furthermore, we demonstrate that Bacteroides colonization exacerbates Aβ deposition, which may be a mechanism whereby the gut impacts AD pathogenesis. These results suggest that long-term CR may alter the gut environment and prevent the expansion of microbes that contribute to age-related cognitive decline.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

