Altered generation of ciliated cells in chronic obstructive pulmonary disease
- PMID: 31784664
- PMCID: PMC6884487
- DOI: 10.1038/s41598-019-54292-x
Altered generation of ciliated cells in chronic obstructive pulmonary disease
Abstract
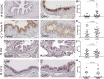
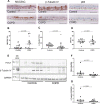
In COPD, epithelial changes are prominent features in the airways, such as goblet cell hyperplasia and squamous metaplasia. In contrast, it remains unclear whether ciliated cells are reduced and which pathways dysregulate epithelial differentiation. We hypothesized that bronchial epithelial cell lineage specification is dysregulated in COPD because of an aberrant reprogramming through transforming growth factor (TGF)-β1. Surgical lung tissue from 81 COPD and 61 control (smokers and non-smokers) patients was assessed for bronchial epithelial cell phenotyping by immunohistochemistry, both in situ and in vitro in reconstituted air-liquid interface (ALI) cultures. The role of TGF-β1 was studied in vitro. COPD epithelium in large airways, when compared to controls, showed decreased β-tubulin IV + ciliated cells (4.4%, 2.5-8.8% versus 8.5%, 6.3-11.8% of surface staining, median and IQR, p = 0.0009) and increased MUC5AC + goblet cells (34.8%, 24.4-41.9% versus 10.3%, 5.1-17.6%, p < 0.0001). Both features were recapitulated in the ALI-cultured epithelium from COPD patients. Exogenous TGF-β1 reduced mucociliary differentiation while neutralizing TGF-β1 during ALI increased both specialized cell types. The COPD airway epithelium displays altered differentiation for ciliated cells, which recapitulates in vitro, at least in part through TGF-β1.
Conflict of interest statement
The authors declare no competing interests.
Figures


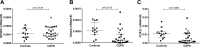


References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, M., and Prevention of COPD, updated, http://www.goldcopd.org (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

