Probing ECM remodeling in idiopathic pulmonary fibrosis via second harmonic generation microscopy analysis of macro/supramolecular collagen structure
- PMID: 31785093
- PMCID: PMC7008503
- DOI: 10.1117/1.JBO.25.1.014505
Probing ECM remodeling in idiopathic pulmonary fibrosis via second harmonic generation microscopy analysis of macro/supramolecular collagen structure
Abstract
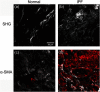
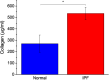
Idiopathic pulmonary fibrosis (IPF) is a progressive disease with poor prognosis with short lifespan following diagnosis as patients have limited effective treatment options. A fundamental limitation is a lack of knowledge of the underlying collagen alterations in the disease, as this could lead to better diagnostics, prognostics, and measures of treatment efficacy. While the fibroses is the primary presentation of the disease, the collagen architecture has not been well studied beyond standard histology. Here, we used several metrics based on second harmonic generation (SHG) microscopy and optical scattering measurements to characterize the subresolution collagen assembly in human IPF and normal lung tissues. Using SHG directional analysis, we found that while collagen synthesis is increased in IPF, the resulting average fibril architecture is more disordered than in normal tissue. Wavelength-dependent optical scattering measurements lead to the same conclusion, and both optical approaches are consistent with ultrastructural analysis. SHG circular dichroism revealed significant differences in the net chirality between the fibrotic and normal collagen, where the former has a more randomized helical structure. Collectively, the measurements reveal significant changes in the collagen macro/supramolecular structure in the abnormal fibrotic collagen, and we suggest these alterations can serve as biomarkers for IPF diagnosis and progression.
Keywords: collagen; fibrosis; polarization; scattering; second harmonic generation.
Figures




Similar articles
-
Examining lysyl oxidase-like modulation of collagen architecture in 3D spheroid models of idiopathic pulmonary fibrosis via second-harmonic generation microscopy.J Biomed Opt. 2021 Jun;26(6):066501. doi: 10.1117/1.JBO.26.6.066501. J Biomed Opt. 2021. PMID: 34145800 Free PMC article.
-
Second harmonic generation microscopy analysis of extracellular matrix changes in human idiopathic pulmonary fibrosis.J Biomed Opt. 2014 Aug;19(8):086014. doi: 10.1117/1.JBO.19.8.086014. J Biomed Opt. 2014. PMID: 25134793 Free PMC article.
-
Stromal alterations in ovarian cancers via wavelength dependent Second Harmonic Generation microscopy and optical scattering.BMC Cancer. 2017 Feb 6;17(1):102. doi: 10.1186/s12885-017-3090-2. BMC Cancer. 2017. PMID: 28166758 Free PMC article.
-
Molecular and tissue alterations of collagens in fibrosis.Matrix Biol. 2018 Aug;68-69:122-149. doi: 10.1016/j.matbio.2018.02.004. Epub 2018 Feb 17. Matrix Biol. 2018. PMID: 29458139 Review.
-
Examination of Collagen Structure and State by the Second Harmonic Generation Microscopy.Biochemistry (Mosc). 2019 Jan;84(Suppl 1):S89-S107. doi: 10.1134/S0006297919140062. Biochemistry (Mosc). 2019. PMID: 31213197 Review.
Cited by
-
Assessment of Ultra-Early-Stage Liver Fibrosis in Human Non-Alcoholic Fatty Liver Disease by Second-Harmonic Generation Microscopy.Int J Mol Sci. 2022 Mar 20;23(6):3357. doi: 10.3390/ijms23063357. Int J Mol Sci. 2022. PMID: 35328778 Free PMC article.
-
Leveraging Optical Anisotropy of the Morpho Butterfly Wing for Quantitative, Stain-Free, and Contact-Free Assessment of Biological Tissue Microstructures.Adv Mater. 2025 Mar;37(12):e2407728. doi: 10.1002/adma.202407728. Epub 2025 Jan 15. Adv Mater. 2025. PMID: 39811986 Free PMC article.
-
Multiscale anisotropy analysis of second-harmonic generation collagen imaging of mouse skin.J Biomed Opt. 2021 Jun;26(6):065002. doi: 10.1117/1.JBO.26.6.065002. J Biomed Opt. 2021. PMID: 34159763 Free PMC article.
-
Lung extracellular matrix modulates KRT5+ basal cell activity in pulmonary fibrosis.Nat Commun. 2023 Sep 27;14(1):6039. doi: 10.1038/s41467-023-41621-y. Nat Commun. 2023. PMID: 37758700 Free PMC article.
-
Generation of a novel lung fibrosis model using precision-cut lung slices from transgenic TGFβ1 mice.Am J Physiol Cell Physiol. 2025 Aug 1;329(2):C611-C623. doi: 10.1152/ajpcell.00015.2025. Epub 2025 Jul 21. Am J Physiol Cell Physiol. 2025. PMID: 40691021 Free PMC article.
References
-
- Campbell K. R., Campagnola P. J., “Wavelength-dependent second harmonic generation circular dichroism for differentiation of Col I and Col III isoforms in stromal models of ovarian cancer based on intrinsic chirality differences,” J. Phys. Chem. B 121, 1749–1757 (2017).JPCBFK10.1021/acs.jpcb.6b06822 - DOI - PMC - PubMed

