Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis
- PMID: 31785173
- PMCID: PMC6885001
- DOI: 10.1002/14651858.CD001176.pub5
Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis
Abstract
Background: Pouchitis occurs in approximately 50% of patients following ileal pouch-anal anastomosis (IPAA) for chronic ulcerative colitis (UC).
Objectives: The primary objective was to determine the efficacy and safety of medical therapies for prevention or treatment of acute or chronic pouchitis.
Search methods: We searched MEDLINE, Embase and CENTRAL from inception to 25 July 2018. We also searched references, trials registers, and conference proceedings.
Selection criteria: Randomized controlled trials of prevention or treatment of acute or chronic pouchitis in adults who underwent IPAA for UC were considered for inclusion.
Data collection and analysis: Two authors independently screened studies for eligibility, extracted data and assessed the risk of bias. The certainty of the evidence was evaluated using GRADE. The primary outcome was clinical improvement or remission in participants with acute or chronic pouchitis, or the proportion of participants with no episodes of pouchitis after IPAA. Adverse events (AEs) was a secondary outcome. We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for each dichotomous outcome.
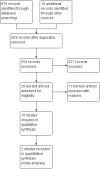
Main results: Fifteen studies (547 participants) were included. Four studies assessed treatment of acute pouchitis. Five studies assessed treatment of chronic pouchitis. Six studies assessed prevention of pouchitis. Three studies were low risk of bias. Three studies were high risk of bias and the other studies were unclear. Acute pouchitis: All ciprofloxacin participants (7/7) achieved remission at two weeks compared to 33% (3/9) of metronidazole participants (RR 2.68, 95% CI 1.13 to 6.35, very low certainty evidence). No ciprofloxacin participants (0/7) had an AE compared to 33% (3/9) of metronidazole participants (RR 0.18, 95% CI 0.01 to 2.98; very low certainty evidence). AEs included vomiting, dysgeusia or transient peripheral neuropathy. Forty-three per cent (6/14) of metronidazole participants achieved remission at 6 weeks compared to 50% (6/12) of budesonide enema participants (RR 0.86, 95% CI 0.37 to 1.96, very low certainty evidence). Fifty per cent (7/14) of metronidazole participants improved clinically at 6 weeks compared to 58% (7/12) of budesonide enema participants (RR 0.86, 95% CI 0.42 to 1.74, very low certainty evidence). Fifty-seven per cent (8/14) of metronidazole participants had an AE compared to 25% (3/12) of budesonide enema participants (RR 2.29, 95% CI 0.78 to 6.73, very low certainty evidence). AEs included anorexia, nausea, headache, asthenia, metallic taste, vomiting, paraesthesia, and depression. Twenty-five per cent (2/8) of rifaximin participants achieved remission at 4 weeks compared to 0% (0/10) of placebo participants (RR 6.11, 95% CI 0.33 to 111.71, very low certainty evidence). Thirty-eight per cent (3/8) of rifaximin participants improved clinically at 4 weeks compared to 30% (3/10) of placebo participants (RR 1.25, 95% CI 0.34 to 4.60, very low certainty evidence). Seventy-five per cent (6/8) of rifaximin participants had an AE compared to 50% (5/10) of placebo participants (RR 1.50, 95% CI 0.72 to 3.14, very low certainty evidence). AEs included diarrhea, flatulence, nausea, proctalgia, vomiting, thirst, candida, upper respiratory tract infection, increased hepatic enzyme, and cluster headache. Ten per cent (1/10) of Lactobacillus GG participants improved clinically at 12 weeks compared to 0% (0/10) of placebo participants (RR 3.00, 95% CI 0.14 to 65.90, very low certainty evidence). Chronic pouchitis: Eighty-five per cent (34/40) of De Simone Formulation (a probiotic formulation) participants maintained remission at 9 to 12 months compared to 3% (1/36) of placebo participants (RR 20.24, 95% CI 4.28 to 95.81, 2 studies; low certainty evidence). Two per cent (1/40) of De Simone Formulation participants had an AE compared to 0% (0/36) of placebo participants (RR 2.43, 95% CI 0.11 to 55.89; low certainty evidence). AEs included abdominal cramps, vomiting and diarrhea. Fifty per cent (3/6) of adalimumab patients achieved clinical improvement at 4 weeks compared to 43% (3/7) of placebo participants (RR, 1.17, 95% CI 0.36 to 3.76, low certainty evidence). Sixty per cent (6/10) of glutamine participants maintained remission at 3 weeks compared to 33% (3/9) of butyrate participants (RR 1.80, 95% CI 0.63 to 5.16, very low certainty evidence). Forty-five per cent (9/20) of patients treated with bismuth carbomer foam enema improved clinically at 3 weeks compared to 45% (9/20) of placebo participants (RR 1.00, 95% CI 0.50 to 1.98, very low certainty evidence). Twenty-five per cent (5/20) of participants in the bismuth carbomer foam enema group had an AE compared to 35% (7/20) of placebo participants (RR 0.71, 95% CI 0.27 to 1.88, very low certainty evidence). Adverse events included diarrhea, worsening symptoms, cramping, sinusitis, and abdominal pain.
Prevention: At 12 months, 90% (18/20) of De Simone Formulation participants had no episodes of acute pouchitis compared to 60% (12/20) of placebo participants (RR 1.50, 95% CI 1.02 to 2.21, low certainty evidence). Another study found 100% (16/16) of De Simone Formulation participants had no episodes of acute pouchitis at 12 months compared to 92% (11/12) of the no treatment control group (RR 1.10, 95% 0.89 to 1.36, very low certainty evidence). Eighty-six per cent (6/7) of Bifidobacterium longum participants had no episodes of acute pouchitis at 6 months compared to 60% (3/5) of placebo participants (RR 1.43, 95% CI 0.66 to 3.11, very low certainty evidence). Eleven per cent (1/9) of Clostridium butyricum MIYAIRI participants had no episodes of acute pouchitis at 24 months compared to 50% (4/8) of placebo participants (RR 0.22, 95% CI 0.03 to 1.60, very low certainty evidence). Forty-six per cent (43/94) of allopurinol participants had no episodes of pouchitis at 24 months compared to 43% (39/90) of placebo participants (RR 1.06, 95% CI 0.76 to 1.46; low certainty evidence). Eighty-one per cent (21/26) of tinidazole participants had no episodes of pouchitis over 12 months compared to 58% (7/12) of placebo participants (RR 1.38, 95% CI 0.83 to 2.31, very low certainty evidence).
Authors' conclusions: The effects of antibiotics, probiotics and other interventions for treating and preventing pouchitis are uncertain. Well designed, adequately powered studies are needed to determine the optimal therapy for the treatment and prevention of pouchitis.
Antecedentes: La reservoritis ocurre en aproximadamente el 50% de los pacientes después de la anastomosis entre la bolsa ileal y el ano (IPAA, por sus siglas en inglés) para la colitis ulcerosa crónica (CU).
Objetivos: El objetivo primario fue determinar la eficacia y la seguridad de los tratamientos médicos para la prevención o el tratamiento de la reservoritis aguda o crónica. MÉTODOS DE BÚSQUEDA: Se hicieron búsquedas en MEDLINE, Embase y en CENTRAL, desde su inicio hasta el 25 julio 2018. También se buscó en las listas de referencias, registros de ensayos en curso y actas de congresos. CRITERIOS DE SELECCIÓN: Se consideraron para inclusión los ensayos controlados aleatorios de prevención o tratamiento de la reservoritis aguda o crónica en adultos a los que se les realiza IPAA para la CU. OBTENCIÓN Y ANÁLISIS DE LOS DATOS: Dos autores de la revisión, de forma independiente, evaluaron la elegibilidad de los estudios, extrajeron los datos y analizaron el riesgo de sesgo. La calidad de la evidencia se evaluó mediante los criterios GRADE. El resultado primario la mejoría clínica o remisión en los pacientes con reservoritis aguda o crónica, o la proporción de pacientes sin episodios de reservoritis después de IPAA. Se incluyeron los eventos adversos como resultado secundario. Se calculó el cociente de riesgos (CR) y el intervalo de confianza (IC) del 95% correspondiente para los resultados dicotómicos.
Resultados principales: Se incluyeron 15 estudios (547 participantes). Cuatro estudios evaluaron el tratamiento de la reservoritis aguda. Cinco estudios evaluaron el tratamiento de la reservoritis crónica. Seis estudios evaluaron la prevención de la reservoritis. Tres estudios presentaban bajo de riesgo de sesgo. En tres estudios el riesgo fue alto y en los otros estudios fue poco claro. reservoritis aguda: Todos los pacientes que recibieron ciprofloxacina (7/7) lograron la remisión a las dos semanas en comparación con el 33% (3/9) de los pacientes que recibieron metronidazol (CR 2,68; IC del 95%: 1,13 a 6,35) (evidencia de certeza muy baja). Ninguno de los participantes que recibieron ciprofloxacina (0/7) presentó eventos adversos en comparación con el 33% (3/9) de los participantes que recibieron metronidazol (CR0,18; IC del 95%: 0,01 a 2,98; evidencia de certeza muy baja). Los eventos adversos incluyeron vómitos, disgeusia o neuropatía periférica transitoria. El 40% (6/14) de los participantes que recibieron metronidazol lograron la remisión a las 6 semanas en comparación con el 50% (6/12) de los participantes que recibieron enema de budesonida (CR 0,86; IC del 95%: 0,37 a 1,96; evidencia de certeza muy baja). El 50% (7/14) de los participantes del grupo de metronidazol mejoraron clínicamente a las 6 semanas en comparación con el 58% (7/12) de los participantes que recibieron enema de budesonida (CR 0,86; IC del 95%: 0,42 a 1,74; evidencia de certeza muy baja). El 57% (8/14) de los participantes del grupo de metronidazol presentaron eventos adversos en comparación con el 25% (3/12) de los participantes que recibieron enema de budesonida (CR 2,29; IC del 95%: 0,78 a 6,73; evidencia de certeza muy baja). Los eventos adversos incluyeron anorexia, náuseas, cefalea, astenia, sabor metálico, vómitos, parestesia y depresión. El 25% (2/8) de los participantes que recibieron rifaximina lograron la remisión a las 4semanas en comparación con el 0% (0/10) de los participantes que recibieron placebo (CR 6,11; IC del 95%: 0,33 a 111,71; evidencia de certeza muy baja). El 38% (3/8) de los participantes del grupo de rifaximina mejoraron clínicamente a las 4 semanas en comparación con el 30% (3/10) de los participantes que recibieron placebo (CR 1,25; IC del 95%: 0,34 a 4,60; evidencia de certeza muy baja). El 75% (6/8) de los participantes del grupo de rifaximina presentaron un evento adverso en comparación con el 50% (5/10) de los participantes que recibieron placebo (CR 1,50; IC del 95%: 0,72 a 3,14; evidencia de certeza muy baja). Los eventos adversos incluyeron diarrea, flatulencias, náuseas, proctalgia, vómitos, sed, cándida, infección de las vías respiratorias superiores, aumento de las enzimas hepáticas y cefalea en racimos. El 10% (1/10) de los participantes del grupo de Lactobacillus GGmejoraron clínicamente a las 12 semanas en comparación con el 0% (0/10) de los participantes que recibieron placebo (CR 3,00; IC del 95%: 0,14 a 65,90; evidencia de certeza muy baja). Reservoritis crónica: El 85% (34/40) de los pacientes que recibieron la formulación De Simone mantuvieron la remisión de nueve a 12 meses en comparación con el 3% (1/36) de los participantes que recibieron placebo (CR 20,24; IC del 95%: 4,28 a 95,81; dos estudios; evidencia de certeza baja). El 2% (1/40) de los participantes que recibieron la fórmula De Simone presentaron un evento adverso, en comparación con el 0% (0/36) de los participantes que recibieron placebo (CR 2,43; IC del 95%: 0,11 a 55,89; evidencia de certeza baja). Los efectos secundarios incluyeron cólicos abdominales, vómitos y diarrea. Cuarenta y tres por ciento (3/6) de los pacientes en el grupo de adalimumab lograron una mejoría clínica a las 4 semanas en comparación con un 43 (3/7) de los pacientes del grupo de placebo (CR 1,17, IC del 95%: 0,36 a 3,76; evidencia de certeza baja). El 60% (6/10) de los participantes del grupo de glutamina mantuvieron la remisión a las 3 semanas en comparación con el 33% (3/9) de los participantes que recibieron placebo (CR 1,80; IC del 95%: 0,63 a 5,16; evidencia de certeza muy baja). El 45% (9/20) de los participantes del grupo de enema de espuma de carbómero de bismuto mejoraron clínicamente a las 3 semanas en comparación con el 45% (9/20) de los participantes que recibieron placebo (CR 1,00; IC del 95%: 0,50 a 1,98; evidencia de certeza muy baja). El 25% (5/20) de los participantes del grupo de aceite de cannabis presentaron un evento adverso en comparación con el 35% (7/20) de los participantes que recibieron placebo (CR 0,71; IC del 95%: 0,27 a 1,88; evidencia de certeza muy baja). Los eventos adversos incluyeron diarrea, síntomas de empeoramiento, cólicos, sinusitis y dolor abdominal. Prevención: A los 12 meses, el 90% (18/20) de los pacientes que recibieron la formulación De Simone no presentaron episodios de reservoritis aguda en comparación con el 60% (12/20) de los pacientes que recibieron placebo (CR 1,50: IC del 95%: 1,02 a 2,21; evidencia de certeza baja). Otro estudio halló que el 100% (16/16) de los participantes que recibieron la fórmula De Simone no presentaron episodios de reservoritis aguda a los 12 meses en comparación con el 92% (11/12) de los pacientes del grupo control sin tratamiento (CR 1,10: IC del 95%: 0,89 a 1,36; evidencia de certeza muy baja). El 86% (6/7) de los participantes del grupo de Bifidobacterium longum no presentaron episodios de reservoritis aguda a los 6 meses en comparación con el 60% (3/5) de los participantes que recibieron placebo (CR 1,43; IC del 95%: 0,66 a 3,11; evidencia de certeza muy baja). El 11% (1/9) de los participantes del grupo de Clostridium butyricum MIYAIRI no presentaron episodios de reservoritis aguda a los 24 meses en comparación con el 50% (4/8) de los participantes que recibieron placebo (CR 0,22; IC del 95%: 0,03 a 1,60; evidencia de certeza muy baja). El 46% (43/94) de los participantes del grupo de alopurinol no presentaron episodios de reservoritis a los 24 meses en comparación con el 43% (39/90) de los participantes que recibieron placebo (CR1,06; IC del 95%: 0,76 a 1,46; evidencia de certeza baja). El 81% (21/26) de los participantes del grupo de tinidazol no presentaron episodios de reservoritis a los 12 meses en comparación con el 58% (7/12) de los participantes que recibieron placebo (CR 1,38; IC del 95%: 0,83 a 2,31; evidencia de certeza muy baja).
Conclusiones de los autores: No se conocen los efectos de los antibióticos, probióticos y otras intervenciones para el tratamiento y la prevención de la reservoritis. Se necesitan estudios bien diseñados con poder estadístico suficiente para determinar la forma óptima de tratamiento y prevención de la reservoritis.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Nghia Nguyen: None known
Bing Zhang: None known
Stefan D Holubar: None known
Darrell S Pardi: has received consulting fees from Assembly Bioscience, C3 Jian, Ferring, Janssen, Nestle, Merck, Otsuka, Salix, Seres, Gilead; research grants from Atlantic, Finch, Janssen, Merck, Pfizer, Salix, Seres, Takeda, Vedanta
Siddharth Singh ‐ has received consulting fees from AbbVie, Takeda, AMAG Pharmaceuticals; research grants from Pfizer and AbbVie; and honorarium for grant review from Pfizer.
Figures






















Update of
-
Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis.Cochrane Database Syst Rev. 2019 May 28;5(5):CD001176. doi: 10.1002/14651858.CD001176.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2019 Nov 30;11:CD001176. doi: 10.1002/14651858.CD001176.pub5. PMID: 31136680 Free PMC article. Updated.
References
References to studies included in this review
Brown 2004 {published data only}
-
- Brown SJ, Megan J, Smith S, Matchet D, Elliott R. Bifidobacterium longum BB‐536 and prevention of acute pouchitis. Gastroenterology 2004;126(4 Suppl 2):S465.
Gionchetti 2000 {published data only}
-
- Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double‐blind, placebo‐controlled trial. Gastroenterology 2000;119(2):305‐9. - PubMed
Gionchetti 2003 {published data only}
-
- Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double‐blind, placebo‐controlled trial. Gastroenterology 2003;124(5):1202‐9. - PubMed
Ha 2010 {published data only}
-
- Ha CY, Bauer JJ, Lazarev M, Swaminath A, Sparrow M, Murphy SJ, et al. Early institution of tinidazole may prevent pouchitis following ileal pouch‐anal anastomosis (IPAA) surgery in ulcerative colitis (UC) patients. Gastroenterology 2010;138(5 Suppl 1):S69.
Isaacs 2007 {published data only}
-
- Isaacs KL, Sandler RS, Abreu M, Picco MF, Hanauer SB, Bickston SJ, et al. Rifaximin for the treatment of active pouchitis: a randomized, double‐blind, placebo‐controlled pilot study. Inflammatory Bowel Diseases 2007;13(10):1250‐5. - PubMed
Joelsson 2001 {published data only}
-
- Joelsson M, Andersson M, Bark T, Gullberg K, Hallgren T, Jiborn H, et al. Allopurinol as prophylaxis against pouchitis following ileal pouch‐anal anastomosis for ulcerative colitis. A randomized placebo‐controlled double‐blind study. Scandinavian Journal of Gastroenterology 2001;36(11):1179‐84. - PubMed
Kjaer 2019 {published data only}
-
- Kjaer MD, Qvist N, Nordgaard‐Lassen I, Christensen LA, Kjeldsen J. Adalimumab in the treatment of chronic pouchitis. A randomized double‐blind, placebo‐controlled trial. Scandinavian Journal of Gastroenterology 2019;54(2):188‐93. - PubMed
Kuisma 2003 {published data only}
-
- Kuisma J, Mentula S, Jarvinen H, Kahri A, Saxelin M, Farkkila M. Effect of Lactobacillus rhamnosus GG on ileal pouch inflammation and microbial flora. Alimentary Pharmacology and Therapeutics 2003;17(4):509‐15. - PubMed
Mimura 2004 {published data only}
Pronio 2008 {published data only}
-
- Pronio A, Montesani C, Butteroni C, Vecchione S, Mumolo G, Vestri A, et al. Probiotic administration in patients with ileal pouch‐anal anastomosis for ulcerative colitis is associated with expansion of mucosal regulatory cells. Inflammatory Bowel Diseases 2008;14(5):662‐8. - PubMed
Sambuelli 2002 {published data only}
-
- Sambuelli A, Boerr L, Negreira S, Gil A, Camartino G, Huernos S, et al. Budesonide enema in pouchitis ‐ A double‐blind, double‐dummy, controlled trial. Alimentary Pharmacology and Therapeutics 2002;16(1):27‐34. - PubMed
Shen 2001 {published data only}
-
- Shen B, Achkar JP, Lashner BA, Ormsby A, Remzi F, Bevins CL, et al. Ciprofloxacin is more effective in treating acute pouchitis than metronidazole: A randomized clinical trial. Gastroenterology 2001;120(5 Suppl 1):A453.
Tremaine 1997 {published data only}
-
- Tremaine WJ, Sandborn WJ, Wolff BG, Carpenter HA, Zinsmeister AR, Metzger PP. Bismuth carbomer foam enemas for active chronic pouchitis: a randomized, double‐blind, placebo‐controlled trial. Alimentary Pharmacology and Therapeutics 1997;11(6):1041‐6. - PubMed
Wischmeyer 1993 {published data only}
-
- Wischmeyer P, Pemberton JH, Phillips SF. Chronic pouchitis after ileal pouch‐anal anastomosis: responses to butyrate and glutamine suppositories in a pilot study. Mayo Clinic Proceedings 1993;68(10):978‐81. - PubMed
Yasueda 2016 {published data only}
-
- Yasueda A, Mizushima T, Nezu R, Sumi R, Tanaka M, Nishimura J, et al. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis.. Surgery Today 2016;46(8):939‐49. [PUBMED: 26510664] - PubMed
References to studies excluded from this review
Bengtsson 2016 {published data only}
-
- Bengtsson J, Adlerberth I, Östblom A, Saksena P, Öresland T, Börjesson L. Effect of probiotics (Lactobacillus plantarum 299 plus Bifidobacterium Cure21) in patients with poor ileal pouch function: a randomised controlled trial.. Scandinavian Journal of Gastroenterology 2016;51(9):1087‐92. [PUBMED: 27150635 ] - PubMed
Kuhbacher 2006 {published data only}
Laake 2005 {published data only}
-
- Laake KO, Bjørneklett A, Aamodt G, Aabakken L, Jacobsen M, Bakka A, et al. Outcome of four weeks' intervention with probiotics on symptoms and endoscopic appearance after surgical reconstruction with a J‐configurated ileal‐pouch‐anal‐anastomosis in ulcerative colitis. Scandinavian Journal of Gastroenterology 2005;40(1):43‐51. - PubMed
Madden 1994 {published data only}
-
- Madden MV, McIntyre AS, Nicholls RJ. Double‐blind crossover trial of metronidazole versus placebo in chronic unremitting pouchitis. Digestive Diseases and Sciences 1994;39(6):1193‐6. - PubMed
McLeod 1986 {published data only}
-
- McLeod RS, Taylor DW, Cohen Z, Cullen JB. Single‐patient randomised clinical trial. Use in determining optimum treatment for patients with inflammation of Kock continent ileostomy reservoir. Lancet 1986;1(8483):726‐8. - PubMed
Meijer 2000 {published data only}
-
- Meijer HP, Welters CF, Heineman E, Salomons GS, Buller HA, Dekker J, et al. Enteral inulin doles not affect epithelial gene expression and cell turnover within the ileoanal pouch ‐ Randomized, placebo‐controlled, crossover study. Diseases of the Colon and Rectum 2000;43(10):1427‐34. - PubMed
Miner 2006 {published data only}
-
- Miner PB Jr, Wedel MK, Xia S, Baker BF. Safety and efficacy of two dose formulations of alicaforsen enema compared with mesalazine enema for treatment of mild to moderate left‐sided ulcerative colitis: A randomized, double‐blind, active‐controlled trial. Alimentary Pharmacology and Therapeutics 2006;23(10):1403‐13. - PubMed
Tomasz 2014 {published data only}
van Assche 2012 {published data only}
-
- Assche G, Ferrante M, Vermeire S, Noman M, Rans K, Biest L, et al. Octreotide for the treatment of diarrhoea in patients with ileal pouch‐anal anastomosis: a placebo‐controlled crossover study. Colorectal Diseases 2012;14(4):181‐6. - PubMed
van Deventer 2006 {published data only}
-
- Deventer SJ, Wedel MK, Baker BF, Xia S, Chuang E, Miner PB Jr. A Phase II dose ranging, double‐blind, placebo‐controlled study of alicaforsen enema in subjects with acute exacerbation of mild to moderate left‐sided ulcerative colitis. Alimentary Pharmacology and Therapeutics 2006;23(10):1415‐25. - PubMed
Welters 2002 {published data only}
-
- Welters CF, Heineman E, Thunnissen FB, Bogaard AE, Soeters PB, Baeten CG. Effect of dietary inulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch‐anal anastomosis. Diseases of the Colon and Rectum 2002;45(5):621‐7. - PubMed
Additional references
Boerr 1995
-
- Boerr LA, Sambuelli AM, Sugai E, Graziano A, Valero J, Kogan Z, et al. Faecal alpha‐1‐antitrypsin concentration in the diagnosis and management of patients with pouchitis. European Journal of Gastroenterology and Hepatology 1995;7(2):129‐33. - PubMed
Boerr 1996
-
- Boerr LA, Sambuelli AM, Filinger E, Peredo A, Graziano A, Valero J, et al. Increased mucosal levels of leukotriene B4 in pouchitis: evidence for a persistent inflammatory state. European Journal of Gastroenterology and Hepatology 1996;8(1):57‐61. - PubMed
Bonello 1981
-
- Bonello JC, Thow GB, Manson RR. Mucosal enteritis: a complication of the continent ileostomy. Diseases of the Colon and Rectum 1981;24(1):37‐41. - PubMed
Bär 2018
-
- Bär F, Kühbacher T, Dietrich NA, Krause T, Stallmach A, Teich N, et al. Vedolizumab in the treatment of chronic, antibiotic‐dependent or refractory pouchitis.. Alimentary Pharmacology and Therapeutics 2018;47(5):581‐7. [PUBMED: 29266360] - PubMed
Chopra 2006
-
- Chopra A, Pardi DS, Loftus EV Jr, Tremaine WJ, Egan LJ, Faubion WA, et al. Budesonide in the treatment of inflammatory bowel disease: the first year of experience in clinical practice.. Inflammatory Bowel Diseases 2006;12(1):29‐32. - PubMed
Clausen 1992
-
- Clausen MR, Tvede M, Mortensen PB. Short‐chain fatty acids in pouch contents with and without pouchitis after ileal pouch‐anal anastomosis. Gastroenterology 1992;103(4):1144‐53. - PubMed
De Silva 1989
-
- Silva HJ, Ireland A, Kettlewell M, Mortensen N, Jewell DP. Short‐chain fatty acid irrigation in severe pouchitis. New England Journal of Medicine 1989;321(20):416‐7. - PubMed
den Hoed 1996
Di Febo 1990
-
- Febo G, Miglioli M, Lauri A, Biasco G, Paganelli GM, Poggioli G, et al. Endoscopic assessment of acute inflammation of the reservoir after restorative ileoanal anastomosis. Gastrointestinal Endoscopy 1990;36(1):6‐9. - PubMed
Gionchetti 1997
-
- Gionchetti P, Rizzello F, Venturi A, Ferretti M, Brignola C, Peruzzo S, et al. Long‐term efficacy of bismuth carbomer enemas in patients with treatment‐resistant chronic pouchitis. Alimentary Pharmacology and Therapeutics 1997;11(4):673‐8. - PubMed
Gionchetti 2007
-
- Gionchetti P, Rizzello F, Poggioli G, Pierangeli F, Laureti S, Morselli C, et al. Oral budesonide in the treatment of chronic refractory pouchitis. Alimentary Pharmacology and Therapeutics 2000;25(10):1231‐6. - PubMed
Gupta 1997
-
- Gupta I, Parihar GA, Malhotra P, Singh GB, Ludtke R, Safayhi H, et al. Effects of Boswella serrata gum resin in patients with ulcerative colitis. European Journal of Medical Research 1997;2(1):37‐43. - PubMed
Guyatt 2008
Herfarth 2015
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hurst 1996
-
- Hurst RD, Molinari M, Chung TP, Rubin M, Michelassi F. Prospective study of incidence, timing, and treatment of pouchitis in 104 consecutive patients after restorative proctocolectomy. Archives of Surgery 1996;131(5):497‐502. - PubMed
Kelly 1983
Klein 1983
-
- Klein K, Stenzel P, Katon RM. Pouch ileitis: report of a case with severe systemic manifestations. Journal of Clinical Gastroenterology 1983;5(2):149‐53. - PubMed
Kmiot 1993
-
- Kmiot WA, Hesslewood SR, Smith N, Thompson H, Harding LK, Keighley MR. Evaluation of the inflammatory infiltrate in pouchitis with 111‐In‐labeled granulocytes. Gastroenterology 1993;104(4):981‐8. - PubMed
Knobler 1986
-
- Knobler H, Ligumsky M, Okon E, Ayalon A, Nesher R, Rachmilewitz D. Pouch ileitis ‐ recurrence of the inflammatory bowel disease in the ileal reservoir. American Journal of Gastroenterology 1986;81(3):199‐201. - PubMed
Levin 1992
-
- Levin KE, Pemberton JH, Phillips SE, Zinsmeister AR, Pezim ME. Role of oxygen free radicals in etiology of pouchitis. Diseases of the Colon and Rectum 1992;35(5):452‐6. - PubMed
Lohmuller 1990
Moskowitz 1986
-
- Moskowitz RL, Shepherd NA, Nicholls RJ. An assessment of inflammation in the reservoir after restorative proctocolectomy with ileoanal ileal reservoir. International Journal of Colorectal Disease 1986;1(3):167‐74. - PubMed
Nasmyth 1989
-
- Nasmyth DG, Godwin PG, Dixon MF, Williams NS, Johnston D. Ileal ecology after pouch‐anal anastomosis or ileostomy. A study of mucosal morphology, fecal bacteriology, fecal volatile fatty acids, and their interrelationship. Gastroenterology 1989;96(3):817‐24. - PubMed
Natori 1992
-
- Natori H, Utsunomiya J, Yamamura T, Benno Y, Uchida K. Fecal and stomal bile acid composition after ileostomy or ileoanal anastomosis in patients with chronic ulcerative colitis and adenomatous coli. Gastroenterology 1992;102(4 Pt 1):1278‐88. - PubMed
Nikfar 2010
-
- Nikfar S, Darvish‐Damavandi M, Abdollahi M. A review and meta‐analysis of the efficacy of antibiotics and probiotics in management of pouchitis. International Journal of Pharmacology 2010;6(6):826‐35.
Pardi 2006
-
- Pardi DS, Sandborn WJ. Systematic review: the management of pouchitis. Alimentary Pharmacology and Therapeutics 2006;23(8):1087‐96. - PubMed
Pardi 2009
-
- Pardi DS, D'Haens G, Shen B, Campbell S, Gionchetti P. Guidelines for the clinical management of pouchitis. Inflammatory Bowel Diseases 2009;15(9):1423‐31. - PubMed
Pullan 1993
Sandborn 1994a
-
- Sandborn WJ. Pouchitis following ileal pouch‐anal anastomosis: definition, pathogenesis, and treatment. Gastroenterology 1994;107(6):1856‐60. - PubMed
Sandborn 1994b
-
- Sandborn WJ, Tremiane WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis following ileal pouch‐anal anastomosis: a pouchitis disease activity index. Mayo Clinic Proceedings 1994;69(5):409‐15. - PubMed
Sandborn 1995
-
- Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Rossi SS, Hofmann AF, et al. Fecal bile acids, short chain fatty acids, and bacteria after ileal pouch‐anal anastomosis do not differ in patients with pouchitis. Digestive Diseases and Sciences 1995;40(7):1474‐83. - PubMed
Sandborn 1997
-
- Sandborn WJ. Pouchitis: definition, risk factors, frequency, natural history, classification, and public health perspectives. In: McLeod RS, Martin F, Sutherland LR, Wallace JL, Williams CN editor(s). Trends In Inflammatory Bowel Disease 1996. Lancaster, UK: Kluwer Academic Publishers, 1997:51‐63.
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Scott 1989
-
- Scott AD, Phillips RK. Ileitis and pouchitis after colectomy for ulcerative colitis. British Journal of Surgery 1989;76(7):668‐9. - PubMed
Segal 2017
-
- Segal JP, Ding NS, Worley G, Mclaughlin S, Preston S, Faiz OD, Clark SK, Hart AL. Systematic review with meta‐analysis: the management of chronic refractory pouchitis with an evidence‐based treatment algorithm.. Alimentary Pharmacology Therapeutics 2017;45(5):581‐92. [PUBMED: 28008631] - PubMed
Shepherd 1987
Shepherd 1989
-
- Shepherd NA, Hulten L, Tytgat GN, Nicholls RJ, Nasmyth DG, Hill MJ, et al. Pouchitis. International Journal of Colorectal Disease 1989;4(4):205‐29. - PubMed
Svaninger 1993
-
- Svaninger G, Nordgren S, Oresland T, Hulten L. Incidence and characteristics of pouchitis in the Kock continent ilesotomy and the pelvic pouch. Scandinavian Journal of Gastroenterology 1993;28(8):695‐700. - PubMed
Tremaine 1994
-
- Tremaine WJ, Sandborn WJ, Phillips SF, Pemberton JH, Carpenter HA. Short chain fatty acid (SCFA) enema therapy for treatment‐resistant pouchitis following ileal pouch‐anal anastomosis (IPAA) for ulcerative colitis (UC). Gastroenterology 1994;106:A784.
Tremaine 1998
-
- Tremaine WJ, Sandborn WJ, Kenan ML. Bismuth subsalicylate tablets for chronic antibiotic‐resistant pouchitis. Gastroenterology 1998;114(1):A1101.
Tytgat 1988
-
- Tytgat GN, Deventer SJ. Pouchitis. International Journal of Colorectal Disease 1988;3(4):226‐8. - PubMed
Zippi 2013
Zuccaro 1989
-
- Zuccaro G Jr, Fazio VW, Church JW, Lavery IC, Ruderman WB, Farmer RG. Pouch ileitis. Digestive Diseases and Sciences 1989;34(10):1505‐10. - PubMed
References to other published versions of this review
Holubar 2010
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

