Validation Studies for Single Circulating Trophoblast Genetic Testing as a Form of Noninvasive Prenatal Diagnosis
- PMID: 31785788
- PMCID: PMC6904821
- DOI: 10.1016/j.ajhg.2019.11.004
Validation Studies for Single Circulating Trophoblast Genetic Testing as a Form of Noninvasive Prenatal Diagnosis
Abstract
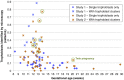
It has long been appreciated that genetic analysis of fetal or trophoblast cells in maternal blood could revolutionize prenatal diagnosis. We implemented a protocol for single circulating trophoblast (SCT) testing using positive selection by magnetic-activated cell sorting and single-cell low-coverage whole-genome sequencing to detect fetal aneuploidies and copy-number variants (CNVs) at ∼1 Mb resolution. In 95 validation cases, we identified on average 0.20 putative trophoblasts/mL, of which 55% were of high quality and scorable for both aneuploidy and CNVs. We emphasize the importance of analyzing individual cells because some cells are apoptotic, in S-phase, or otherwise of poor quality. When two or more high-quality trophoblast cells were available for singleton pregnancies, there was complete concordance between all trophoblasts unless there was evidence of confined placental mosaicism. SCT results were highly concordant with available clinical data from chorionic villus sampling (CVS) or amniocentesis procedures. Although determining the exact sensitivity and specificity will require more data, this study further supports the potential for SCT testing to become a diagnostic prenatal test.
Keywords: cell-based NIPT; fetal; mosaisicm; noninvasive prenatal diagnosis; single cell analysis; trophoblast.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Baylor Genetics (BG) is a diagnostic laboratory partially owned by Baylor College of Medicine. Several authors are located at BG, as indicated, and A.L.B. and I.B.V. have consulting or committee roles at BG. A.L.B. is a founder of Luna Genetics.
Figures



References
-
- Hartwig T.S., Ambye L., Sørensen S., Jørgensen F.S. Discordant non-invasive prenatal testing (NIPT) - a systematic review. Prenat. Diagn. 2017;37:527–539. - PubMed
-
- Neofytou M.C., Tsangaras K., Kypri E., Loizides C., Ioannides M., Achilleos A., Mina P., Keravnou A., Sismani C., Koumbaris G., Patsalis P.C. Targeted capture enrichment assay for non-invasive prenatal testing of large and small size sub-chromosomal deletions and duplications. PLoS ONE. 2017;12:e0171319. - PMC - PubMed
-
- Scott F.P., Menezes M., Palma-Dias R., Nisbet D., Schluter P., da Silva Costa F., McLennan A.C. Factors affecting cell-free DNA fetal fraction and the consequences for test accuracy. J. Matern. Fetal Neonatal Med. 2018;31:1865–1872. - PubMed
-
- Van Opstal D., Srebniak M.I., Polak J., de Vries F., Govaerts L.C.P., Joosten M., Go A.T.J.I., Knapen M.F.C.M., van den Berg C., Diderich K.E.M., Galjaard R.J. Falsenegative NIPT results: Risk figures for chromosomes 13, 18 and 21 based on chorionic villi results in 5967 cases and literature review. PLoS ONE. 2016;11:e0146794. - PMC - PubMed
-
- Krabchi K., Gros-Louis F., Yan J., Bronsard M., Massé J., Forest J.C., Drouin R. Quantification of all fetal nucleated cells in maternal blood between the 18th and 22nd weeks of pregnancy using molecular cytogenetic techniques. Clin. Genet. 2001;60:145–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

