Rewiring Neuronal Glycerolipid Metabolism Determines the Extent of Axon Regeneration
- PMID: 31786011
- PMCID: PMC6975164
- DOI: 10.1016/j.neuron.2019.10.009
Rewiring Neuronal Glycerolipid Metabolism Determines the Extent of Axon Regeneration
Abstract
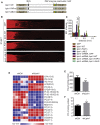
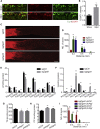
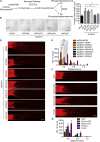
How adult neurons coordinate lipid metabolism to regenerate axons remains elusive. We found that depleting neuronal lipin1, a key enzyme controlling the balanced synthesis of glycerolipids through the glycerol phosphate pathway, enhanced axon regeneration after optic nerve injury. Axotomy elevated lipin1 in retinal ganglion cells, which contributed to regeneration failure in the CNS by favorably producing triglyceride (TG) storage lipids rather than phospholipid (PL) membrane lipids in neurons. Regrowth induced by lipin1 depletion required TG hydrolysis and PL synthesis. Decreasing TG synthesis by deleting neuronal diglyceride acyltransferases (DGATs) and enhancing PL synthesis through the Kennedy pathway promoted axon regeneration. In addition, peripheral neurons adopted this mechanism for their spontaneous axon regeneration. Our study reveals a critical role of lipin1 and DGATs as intrinsic regulators of glycerolipid metabolism in neurons and indicates that directing neuronal lipid synthesis away from TG synthesis and toward PL synthesis may promote axon regeneration.
Keywords: DGAT1; DGAT2; Lipin1; axon regeneration; glycerolipid; phospholipid; retinal ganglion cell; triglyceride.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures





Comment in
-
Greasing the Wheels of Regeneration.Neuron. 2020 Jan 22;105(2):207-209. doi: 10.1016/j.neuron.2019.11.032. Neuron. 2020. PMID: 31972142
References
-
- Aldskogius H. Lipid accumulation in axotomized adult rabbit vagal neurons. Electron microscopical observations. Brain Res. 1978;140:349–353. - PubMed
-
- Bazinet R.P., Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014;15:771–785. - PubMed
-
- Blanquie O., Bradke F. Cytoskeleton dynamics in axon regeneration. Curr. Opin. Neurobiol. 2018;51:60–69. - PubMed
-
- Bloch K. The biological synthesis of cholesterol. Science. 1965;150:19–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

