Non-Invasive Venous waveform Analysis (NIVA) for monitoring blood loss in human blood donors and validation in a porcine hemorrhage model
- PMID: 31786067
- PMCID: PMC8171001
- DOI: 10.1016/j.jclinane.2019.109664
Non-Invasive Venous waveform Analysis (NIVA) for monitoring blood loss in human blood donors and validation in a porcine hemorrhage model
Abstract
Study objective: There is an unmet need for a non-invasive approach to diagnose hemorrhage early, before changes in vital signs occur. Non-Invasive Venous waveform Analysis (NIVA) uses a unique physiological signal (the peripheral venous waveform) to assess intravascular volume. We hypothesized changes in the venous waveform would be observed with blood loss in healthy adult blood donors and characterized hemorrhage using invasive monitoring in a porcine model.
Design: Prospective observational study.
Setting: American Red Cross donation center.
Patients: 50 human blood donors and 12 non-donating controls; 7 Yorkshire pigs.
Interventions: A venous waveform capturing prototype (NIVA device) was secured to the volar aspect of the wrist in human subjects. A central venous catheter was used to obtain hemodynamic indices and venous waveforms were obtained using the prototype NIVA device over the saphenous vein during 400 mL of graded hemorrhage in a porcine model.
Measurements: Venous waveforms were transformed from the time to the frequency domain. The ratiometric power contributions of the cardiac frequencies were used to calculate a NIVA value representative of volume status.
Main results: A significant decrease in NIVA value was observed after 500 mL of whole blood donation (p < .05). A ROC curve for the ability of the NIVA to detect 500 mL of blood loss demonstrated an area under the curve (AUC) of 0.94. In the porcine model, change in NIVA value correlated linearly with blood loss and with changes in hemodynamic indices.
Conclusions: This study provides proof-of-concept for a potential application of NIVA in detection of blood loss. NIVA represents a novel physiologic signal for detection of early blood loss that may be useful in early triage and perioperative management.
Keywords: Hemorrhage; Monitoring; Venous; Venous waveform analysis.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTEREST
Kyle Hocking, PhD, is Founder, CEO and President of VoluMetrix and an inventor on intellectual property in the field of venous waveform analysis assigned to Vanderbilt and licensed to VoluMetrix. Colleen Brophy, MD, is Founder and CMO of VoluMetrix and an inventor on intellectual property in the field of venous waveform analysis assigned to Vanderbilt and licensed to VoluMetrix. Bret Alvis, MD, owns stock in VoluMetrix and an inventor on intellectual property in the field of venous waveform analysis assigned to Vanderbilt and licensed to VoluMetrix and is married to the COO of VoluMetrix.
Figures
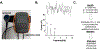





References
-
- Antonelli M, Levy M, Andrews PJ, Chastre J, Hudson LD, Manthous C, et al. Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27–28 April 2006. Intensive Care Med 2007;33:575–90. - PubMed
-
- Newgard CD, Uribe-Leitz T, Haider AH. Undertriage Remains a Vexing Problem for Even the Most Highly Developed Trauma Systems: The Need for Innovations in Field Triage. JAMA Surg 2018;153:328. - PubMed
-
- Haase-Fielitz A, Haase M, Bellomo R, Calzavacca P, Spura A, Baraki H, et al. Perioperative Hemodynamic Instability and Fluid Overload are Associated with Increasing Acute Kidney Injury Severity and Worse Outcome after Cardiac Surgery. Blood Purif 2017;43:298–308. - PubMed
-
- Goepfert MS, Reuter DA, Akyol D, Lamm P, Kilger E, Goetz AE. Goal-directed fluid management reduces vasopressor and catecholamine use in cardiac surgery patients. Intensive Care Med 2007;33:96–103. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

