Neocortex-Cerebellum Circuits for Cognitive Processing
- PMID: 31787351
- PMCID: PMC6942222
- DOI: 10.1016/j.tins.2019.11.002
Neocortex-Cerebellum Circuits for Cognitive Processing
Abstract
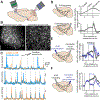
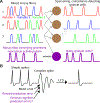
Although classically thought of as a motor circuit, the cerebellum is now understood to contribute to a wide variety of cognitive functions through its dense interconnections with the neocortex, the center of brain cognition. Recent investigations have shed light on the nature of cerebellar cognitive processing and information exchange with the neocortex. We review findings that demonstrate widespread reward-related cognitive input to the cerebellum, as well as new studies that have characterized the codependence of processing in the neocortex and cerebellum. Together, these data support a view of the neocortex-cerebellum circuit as a joint dynamic system both in classical sensorimotor contexts and reward-related, cognitive processing. These studies have also expanded classical theory on the computations performed by the cerebellar circuit.
Keywords: cerebellum; learning; motor control; neocortex; neural dynamics; reward.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

References
-
- Ito M (2000) Mechanisms of motor learning in the cerebellum. Brain research 886, 237–245 - PubMed
-
- Ivry RB, et al. (2002) The cerebellum and event timing. Ann NY Acad Sci 978, 302–317 - PubMed
-
- Bastian AJ (2006) Learning to predict the future: the cerebellum adapts feedforward movement control. Current Opinion in Neurobiology 16, 645–649 - PubMed
-
- Gao Z, et al. (2012) Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci 13, 619. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

