Uncovering the role of MAFB in glucagon production and secretion in pancreatic α-cells using a new α-cell-specific Mafb conditional knockout mouse model
- PMID: 31787710
- PMCID: PMC7220711
- DOI: 10.1538/expanim.19-0105
Uncovering the role of MAFB in glucagon production and secretion in pancreatic α-cells using a new α-cell-specific Mafb conditional knockout mouse model
Abstract
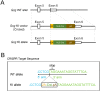
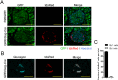
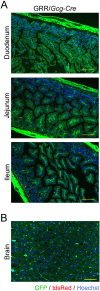
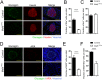
Cre/loxP is a site-specific recombination system extensively used to enable the conditional deletion or activation of target genes in a spatial- and/or temporal-specific manner. A number of pancreatic-specific Cre driver mouse lines have been broadly established for studying the development, function and pathology of pancreatic cells. However, only a few models are currently available for glucagon-producing α-cells. Disagreement exists over the role of the MAFB transcription factor in glucagon expression during postnatal life, which might be due to the lack of α-cell-specific Cre driver mice. In the present study, we established a novel Gcg-Cre knock-in mouse line with the Cre transgene expressed under the control of the preproglucagon (Gcg) promoter without disrupting the endogenous Gcg gene expression. Then, we applied this newly developed Gcg-Cre mouse line to generate a new α-cell-specific Mafb conditional knockout mouse model (MafbΔGcg). Not only α-cell number but also glucagon production were significantly decreased in MafbΔGcg mice compared to control littermates, suggesting an indispensable role of MAFB in both α-cell development and function. Taken together, our newly developed Gcg-Cre mouse line, which was successfully utilized to uncover the role of MAFB in α-cells, is a useful tool for genetic manipulation in pancreatic α-cells, providing a new platform for future studies in this field.
Keywords: Cre/loxP; MAFB; glucagon; pancreatic islet; α-cells.
Figures
Similar articles
-
High-fidelity Glucagon-CreER mouse line generated by CRISPR-Cas9 assisted gene targeting.Mol Metab. 2017 Jan 12;6(3):236-244. doi: 10.1016/j.molmet.2017.01.003. eCollection 2017 Mar. Mol Metab. 2017. PMID: 28271030 Free PMC article.
-
Gcg CreERT2 knockin mice as a tool for genetic manipulation in pancreatic alpha cells.Diabetologia. 2017 Dec;60(12):2399-2408. doi: 10.1007/s00125-017-4425-x. Epub 2017 Sep 7. Diabetologia. 2017. PMID: 28884202 Free PMC article.
-
MafB Is Critical for Glucagon Production and Secretion in Mouse Pancreatic α Cells In Vivo.Mol Cell Biol. 2018 Mar 29;38(8):e00504-17. doi: 10.1128/MCB.00504-17. Print 2018 Apr 15. Mol Cell Biol. 2018. PMID: 29378833 Free PMC article.
-
Metabolic impact of glucagon deficiency.Diabetes Obes Metab. 2011 Oct;13 Suppl 1:151-7. doi: 10.1111/j.1463-1326.2011.01456.x. Diabetes Obes Metab. 2011. PMID: 21824269 Review.
-
Glucagon gene expression in the endocrine pancreas: the role of the transcription factor Pax6 in α-cell differentiation, glucagon biosynthesis and secretion.Diabetes Obes Metab. 2011 Oct;13 Suppl 1:31-8. doi: 10.1111/j.1463-1326.2011.01445.x. Diabetes Obes Metab. 2011. PMID: 21824254 Review.
Cited by
-
Not the second fiddle: α cell development, identity, and function in health and diabetes.J Endocrinol. 2023 Jul 11;258(2):e220297. doi: 10.1530/JOE-22-0297. Print 2023 Aug 1. J Endocrinol. 2023. PMID: 37171828 Free PMC article. Review.
-
Recent advances in pancreatic α-cell transdifferentiation for diabetes therapy.Front Immunol. 2025 Jan 22;16:1551372. doi: 10.3389/fimmu.2025.1551372. eCollection 2025. Front Immunol. 2025. PMID: 39911402 Free PMC article. Review.
-
NKX2.2 and KLF4 cooperate to regulate α-cell identity.Genes Dev. 2025 Feb 3;39(3-4):242-260. doi: 10.1101/gad.352193.124. Genes Dev. 2025. PMID: 39797760 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

