Macrophage-associated pro-inflammatory state in human islets from obese individuals
- PMID: 31787760
- PMCID: PMC6885511
- DOI: 10.1038/s41387-019-0103-z
Macrophage-associated pro-inflammatory state in human islets from obese individuals
Abstract
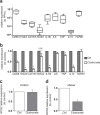
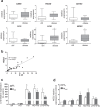
Obesity is associated with inflammatory macrophages in insulin responsive tissues and the resulting inflammatory response is a major contributor to insulin resistance. In insulin-producing pancreatic islets, the intra-islet accumulation of macrophages is observed in patients of type 2 diabetes (T2D), but such has not been investigated in obese individuals. Here, we show that pro-inflammatory cytokines (IL-1β, IL-6, and TNF), anti-inflammatory cytokines (IL-10 and TGF-β) and macrophage polarization markers (CD11c, CD163, and NOS2) were expressed in isolated human islets from non-diabetic donors. Clodronate-mediated depletion of resident macrophages revealed expression of IL1B and IL10 mostly from macrophages, while IL6, TNF, and TGFB1 came largely from a non-macrophage origin in human islets. NOS2 expression came exclusively from non-macrophage cells in non-obese individuals, while it originated also from macrophages in obese donors. Macrophage marker expression of CD68, CD163, and ITGAX was unchanged in islets of non-obese control and obese cohorts. In contrast, IL1B and NOS2 were significantly increased in islets from obese, compared to non-obese individuals, implying a more inflammatory macrophage phenotype in islets in obesity. Our study shows elevated macrophage-associated inflammation in human islets in obesity, which could be an initiating factor to the pro-inflammatory intra-islet milieu and contribute to the higher susceptibility to T2D in obese individuals.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

