Lithium Perchlorate-, Acetic Anhydride-, and Triphenylphosphine-assisted Multicomponent Syntheses of 4-Unsubstituted 2,5-Dioxooctahydroquinoline-3-carboxylates and 3-carbonitriles
- PMID: 31787785
- PMCID: PMC6884338
- DOI: 10.1016/j.tet.2013.08.081
Lithium Perchlorate-, Acetic Anhydride-, and Triphenylphosphine-assisted Multicomponent Syntheses of 4-Unsubstituted 2,5-Dioxooctahydroquinoline-3-carboxylates and 3-carbonitriles
Abstract
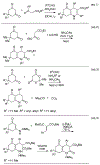
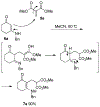
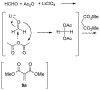
Lithium perchlorate and acetic anhydride were the key additives for the multi-component reaction between 3-aminocyclohex-2-enones, formaldehyde, and malonates yielding adducts that were annulated under acidic conditions to afford bicyclic 2,5-dioxooctahydroquinoline-3-carboxylates. When methyl cyanoacetate was subjected to the same reaction conditions in the presence of a catalytic amount of triphenylphosphine, the bicyclic 2,5-dioxooctahydroquinoline-3-carbonitriles were obtained in a one-flask reaction.
Keywords: 2,5-dioxooctahydroquinolines; Multicomponent reaction; acetic anhydride; catalysis; lithium perchlorate; triphenylphosphine.
Figures

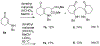
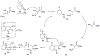
References
Grants and funding
LinkOut - more resources
Full Text Sources
