Bioscaffold-Induced Brain Tissue Regeneration
- PMID: 31787865
- PMCID: PMC6855095
- DOI: 10.3389/fnins.2019.01156
Bioscaffold-Induced Brain Tissue Regeneration
Abstract
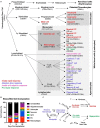
Brain tissue lost after a stroke is not regenerated, although a repair response associated with neurogenesis does occur. A failure to regenerate functional brain tissue is not caused by the lack of available neural cells, but rather the absence of structural support to permit a repopulation of the lesion cavity. Inductive bioscaffolds can provide this support and promote the invasion of host cells into the tissue void. The putative mechanisms of bioscaffold degradation and its pivotal role to permit invasion of neural cells are reviewed and discussed in comparison to peripheral wound healing. Key differences between regenerating and non-regenerating tissues are contrasted in an evolutionary context, with a special focus on the neurogenic response as a conditio sine qua non for brain regeneration. The pivotal role of the immune system in biodegradation and the formation of a neovasculature are contextualized with regeneration of peripheral soft tissues. The application of rehabilitation to integrate newly forming brain tissue is suggested as necessary to develop functional tissue that can alleviate behavioral impairments. Pertinent aspects of brain tissue development are considered to provide guidance to produce a metabolically and functionally integrated de novo tissue. Although little is currently known about mechanisms involved in brain tissue regeneration, this review outlines the various components and their interplay to provide a framework for ongoing and future studies. It is envisaged that a better understanding of the mechanisms involved in brain tissue regeneration will improve the design of biomaterials and the methods used for implantation, as well as rehabilitation strategies that support the restoration of behavioral functions.
Keywords: biodegradation; biomaterial; extracellular matrix; physical therapy; regeneration; scaffold; stroke; tissue repair.
Copyright © 2019 Modo.
Figures





References
-
- Allman A. J., Mcpherson T. B., Badylak S. F., Merrill L. C., Kallakury B., Sheehan C., et al. (2001). Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation 71 1631–1640. - PubMed
-
- Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O. (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8 963–970. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

