17β-Estradiol Attenuates Neuropathic Pain Caused by Spared Nerve Injury by Upregulating CIC-3 in the Dorsal Root Ganglion of Ovariectomized Rats
- PMID: 31787875
- PMCID: PMC6856564
- DOI: 10.3389/fnins.2019.01205
17β-Estradiol Attenuates Neuropathic Pain Caused by Spared Nerve Injury by Upregulating CIC-3 in the Dorsal Root Ganglion of Ovariectomized Rats
Abstract
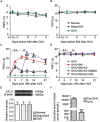
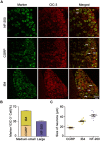
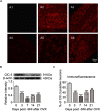
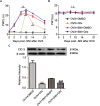
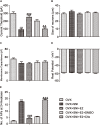
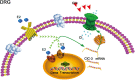
17β-estradiol plays a role in pain sensitivity, analgesic drug efficacy, and neuropathic pain prevalence, but the underlying mechanisms remain unclear. Here, we investigated whether voltage-gated chloride channel-3 (ClC-3) impacts the effects of 17β-estradiol (E2) on spared nerve injury (SNI)-induced neuropathic pain in ovariectomized (OVX) female Sprague Dawley rats that were divided into OVX, OVX + SNI, OVX + SNI + E2, OVX + SNI + E2 + DMSO (vehicle, dimethyl sulfoxide), or OVX + SNI + E2+Cltx (ClC-3-blocker chlorotoxin) groups. Changes in ClC-3 protein expression were monitored by western blot analysis. Behavioral testing used the paw withdrawal threshold to acetone irritation and paw withdrawal thermal latency (PWTL) to thermal stimulation. Immunofluorescence indicated the localization and protein expression levels of ClC-3. OVX + SNI + E2 rats were subcutaneously injected with 17β-estradiol once daily for 7 days; a sheathed tube was implanted, and chlorotoxin was injected for 4 days. Intrathecal Cltx to OVX and OVX + SNI rats was administered for 4 consecutive days (days 7-10 after SNI) to further determine the contribution of ClC-3 to neuropathic pain. Patch clamp technology in current clamp mode was used to measure the current threshold (rheobase) dorsal root ganglion (DRG) neurons and the minimal current that evoked action potentials (APs) as excitability parameters. The mean number of APs at double-strength rheobase verified neuronal excitability. There was no difference in behaviors and ClC-3 expression after OVX. Compared with OVX + SNI rats, OVX + SNI + E2 rats showed a lower paw withdrawal threshold to the acetone stimulus, but the PWTL was not significantly different, indicating increased sensitivity to cold but not to thermal pain. Co-immunofluorescent data revealed that ClC-3 was mainly distributed in A- and C-type nociceptive neurons, especially in medium/small-sized neurons. 17β-estradiol administration was associated with increased expression of ClC-3. 17β-estradiol-induced increase in ClC-3 expression was blocked by co-administration of Cltx. Cltx causes hyperalgesia and decreased expression of ClC-3 in OVX rats. Patch clamp results suggested that 17β-estradiol attenuated the excitability of neurons induced by SNI by up-regulating the expression of ClC-3 in the DRG of OVX rats. 17β-estradiol administration significantly improved cold allodynia thresholds in OVX rats with SNI. The mechanism for this decreased sensitivity may be related to the upregulation of ClC-3 expression in the DRG.
Keywords: 17β-estradiol; ClC-3; neuropathic pain; ovariectomy; spared nerve injury.
Copyright © 2019 Xu, Chen, Deng, Zhang, Tan, Wang, Ma, Li, Si and Zhu.
Figures



Similar articles
-
The Role of TMEM16A/ERK/NK-1 Signaling in Dorsal Root Ganglia Neurons in the Development of Neuropathic Pain Induced by Spared Nerve Injury (SNI).Mol Neurobiol. 2021 Nov;58(11):5772-5789. doi: 10.1007/s12035-021-02520-9. Epub 2021 Aug 18. Mol Neurobiol. 2021. PMID: 34406600 Free PMC article.
-
Microglial BDNF, PI3K, and p-ERK in the Spinal Cord Are Suppressed by Pulsed Radiofrequency on Dorsal Root Ganglion to Ease SNI-Induced Neuropathic Pain in Rats.Pain Res Manag. 2019 Apr 28;2019:5948686. doi: 10.1155/2019/5948686. eCollection 2019. Pain Res Manag. 2019. PMID: 31182984 Free PMC article.
-
Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain.Sheng Li Xue Bao. 2008 Oct 25;60(5):579-80. Sheng Li Xue Bao. 2008. PMID: 18958363
-
Estrogen affects neuropathic pain through upregulating N-methyl-D-aspartate acid receptor 1 expression in the dorsal root ganglion of rats.Neural Regen Res. 2017 Mar;12(3):464-469. doi: 10.4103/1673-5374.202925. Neural Regen Res. 2017. PMID: 28469663 Free PMC article.
-
Downregulation of ClC-3 in dorsal root ganglia neurons contributes to mechanical hypersensitivity following peripheral nerve injury.Neuropharmacology. 2016 Nov;110(Pt A):181-189. doi: 10.1016/j.neuropharm.2016.07.023. Epub 2016 Jul 25. Neuropharmacology. 2016. PMID: 27460962
Cited by
-
Comparison of pain modulatory effect of the LPGi estragon receptor on inflammatory pain between pro-estrus and estrus phases and OVX rats.Psychopharmacology (Berl). 2025 Jan;242(1):129-147. doi: 10.1007/s00213-024-06653-2. Epub 2024 Aug 24. Psychopharmacology (Berl). 2025. PMID: 39180591
-
Improving NKCC1 Function Increases the Excitability of DRG Neurons Exacerbating Pain Induced After TRPV1 Activation of Primary Sensory Neurons.Front Cell Neurosci. 2021 May 25;15:665596. doi: 10.3389/fncel.2021.665596. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34113239 Free PMC article.
-
Novel Analgesics with Peripheral Targets.Neurotherapeutics. 2020 Jul;17(3):784-825. doi: 10.1007/s13311-020-00937-z. Epub 2020 Oct 15. Neurotherapeutics. 2020. PMID: 33063247 Free PMC article. Review.
-
The Role of TMEM16A/ERK/NK-1 Signaling in Dorsal Root Ganglia Neurons in the Development of Neuropathic Pain Induced by Spared Nerve Injury (SNI).Mol Neurobiol. 2021 Nov;58(11):5772-5789. doi: 10.1007/s12035-021-02520-9. Epub 2021 Aug 18. Mol Neurobiol. 2021. PMID: 34406600 Free PMC article.
-
Estrogen receptors in pain modulation: cellular signaling.Biol Sex Differ. 2021 Feb 10;12(1):22. doi: 10.1186/s13293-021-00364-5. Biol Sex Differ. 2021. PMID: 33568220 Free PMC article. Review.
References
-
- Amescua-Garcia C., Colimon F., Guerrero C., Jreige Iskandar A., Berenguel Cook M., Bonilla P., et al. (2018). Most relevant neuropathic pain treatment and chronic low back pain management guidelines: a change pain Latin America advisory panel consensus. Pain Med. 19 460–470. 10.1093/pm/pnx198 - DOI - PubMed
-
- Bálint F., Liposits Z., Farkas I. (2016). Estrogen receptor beta and 2-arachidonoylglycerol mediate the suppressive effects of estradiol on frequency of postsynaptic currents in gonadotropin-releasing hormone neurons of metestrous mice: an acute slice electrophysiological study. Front. Cell. Neurosci. 10:77. 10.3389/fncel.2016.00077 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

