Dual-Species Model Biofilm Consisting of Listeria monocytogenes and Salmonella Typhimurium: Development and Inactivation With Cold Atmospheric Plasma (CAP)
- PMID: 31787943
- PMCID: PMC6854999
- DOI: 10.3389/fmicb.2019.02524
Dual-Species Model Biofilm Consisting of Listeria monocytogenes and Salmonella Typhimurium: Development and Inactivation With Cold Atmospheric Plasma (CAP)
Abstract
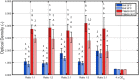
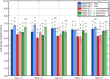
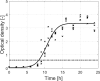
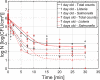
Most environmental biofilms contain a variety of species. These species can establish cooperative and competitive interactions, possibly resulting in an increase or a decrease in antimicrobial resistance. Therefore, results obtained following inactivation of single-species biofilms by means of different technologies (e.g., Cold Atmospheric Plasma, CAP) should be validated for multi-species biofilms. First, a strongly adherent and mature Listeria monocytogenes and S. Typhimurium dual-species biofilm was developed by altering different incubation conditions, i.e., growth medium, incubation temperature, inoculum ratio of L. monocytogenes and S. Typhimurium cells, and incubation time. Adherence and maturity were quantified by means of optical density measurements and viable plate counts, respectively. Secondly, both the (1 day old) reference biofilm and a more mature 7 days old biofilm were treated for different CAP treatment times (0-30 min). Viable plate counts were again used to determine the (remaining) cell density. For both the biofilm development and inactivation, predictive models were applied to describe the growth/inactivation kinetics. Finally, the kinetics of the [1 and 7 day(s) old] dual-species biofilms were compared with those obtained for the corresponding single-species biofilms. Results implied that a strongly adherent and mature reference dual-species biofilm was obtained following 24 h of incubation at 25°C using 20-fold diluted TSB and an inoculum ratio of 1:1. Main observations regarding CAP inactivation were: (i) the dual-species biofilm age had no influence on the CAP efficacy, although a longer treatment time was required for the oldest biofilm, (ii) for the 1 day old biofilms, CAP treatment became less efficient for S. Typhimurium inactivation when this species was part of the dual-species biofilm, while L. monocytogenes inactivation was not influenced by the biofilm type, and (iii) for the 7 days old biofilms, CAP inactivation of both species became more efficient when they were part of the dual-species biofilms. It can be concluded that the efficacy of the CAP treatment is altered when cells become part of a dual-species biofilm, which is quite important with respect to a possible application of CAP for biofilm inactivation within the food industry.
Keywords: L. monocytogenes; S. Typhimurium; biofilms; cold atmospheric plasma (CAP); dual-species; inactivation; single-species.
Copyright © 2019 Govaert, Smet, Walsh and Van Impe.
Figures


Similar articles
-
Inactivation of L. monocytogenes and S. typhimurium Biofilms by Means of an Air-Based Cold Atmospheric Plasma (CAP) System.Foods. 2020 Feb 6;9(2):157. doi: 10.3390/foods9020157. Foods. 2020. PMID: 32041294 Free PMC article.
-
Combined Effect of Cold Atmospheric Plasma and Hydrogen Peroxide Treatment on Mature Listeria monocytogenes and Salmonella Typhimurium Biofilms.Front Microbiol. 2019 Nov 20;10:2674. doi: 10.3389/fmicb.2019.02674. eCollection 2019. Front Microbiol. 2019. PMID: 31824459 Free PMC article.
-
Influence of incubation conditions on the formation of model biofilms by Listeria monocytogenes and Salmonella Typhimurium on abiotic surfaces.J Appl Microbiol. 2018 Dec 1;125(6). doi: 10.1111/jam.14071. Epub 2018 Aug 17. J Appl Microbiol. 2018. PMID: 30117654
-
Fighting Mixed-Species Microbial Biofilms With Cold Atmospheric Plasma.Front Microbiol. 2020 May 20;11:1000. doi: 10.3389/fmicb.2020.01000. eCollection 2020. Front Microbiol. 2020. PMID: 32508796 Free PMC article. Review.
-
Preclinical Cold Atmospheric Plasma Cancer Treatment.Cancers (Basel). 2022 Jul 16;14(14):3461. doi: 10.3390/cancers14143461. Cancers (Basel). 2022. PMID: 35884523 Free PMC article. Review.
Cited by
-
How Good are Bacteriophages as an Alternative Therapy to Mitigate Biofilms of Nosocomial Infections.Infect Drug Resist. 2022 Feb 17;15:503-532. doi: 10.2147/IDR.S348700. eCollection 2022. Infect Drug Resist. 2022. PMID: 35210792 Free PMC article. Review.
-
Understanding the Interactions between Staphylococcus aureus and the Raw-Meat-Processing Environment Isolate Klebsiella oxytoca in Dual-Species Biofilms via Discovering an Altered Metabolic Profile.Microorganisms. 2021 Mar 24;9(4):672. doi: 10.3390/microorganisms9040672. Microorganisms. 2021. PMID: 33805148 Free PMC article.
-
A Hyaluronic Acid Functionalized Self-Nano-Emulsifying Drug Delivery System (SNEDDS) for Enhancement in Ciprofloxacin Targeted Delivery against Intracellular Infection.Nanomaterials (Basel). 2021 Apr 22;11(5):1086. doi: 10.3390/nano11051086. Nanomaterials (Basel). 2021. PMID: 33922241 Free PMC article.
-
Cold Atmospheric Plasma Ameliorates Skin Diseases Involving Reactive Oxygen/Nitrogen Species-Mediated Functions.Front Immunol. 2022 May 26;13:868386. doi: 10.3389/fimmu.2022.868386. eCollection 2022. Front Immunol. 2022. PMID: 35720416 Free PMC article. Review.
-
Behavior of the Surviving Population of Listeria monocytogenes and Salmonella Typhimurium Biofilms Following a Direct Helium-Based Cold Atmospheric Plasma Treatment.Front Microbiol. 2022 Mar 24;13:831434. doi: 10.3389/fmicb.2022.831434. eCollection 2022. Front Microbiol. 2022. PMID: 35401458 Free PMC article.
References
-
- Baka M., Noriega E., Stamati I., Logist F., Van Impe J. F. M. (2015). Critical assessment of the time-to-detection method for accurate estimation of microbial growth parameters. J. Food SAF 35, 179–192. 10.1111/jfs.12170 - DOI
-
- Banu M. S., Sasikala P., Dhanapal A., Kavitha V., Yazhini G., Rajamani L. (2012). Cold plasma as a novel food processing technology. Annu. Rev. 4, 803–818.
LinkOut - more resources
Full Text Sources
Miscellaneous

