Soluble CD137 Ameliorates Acute Type 1 Diabetes by Inducing T Cell Anergy
- PMID: 31787971
- PMCID: PMC6853870
- DOI: 10.3389/fimmu.2019.02566
Soluble CD137 Ameliorates Acute Type 1 Diabetes by Inducing T Cell Anergy
Abstract
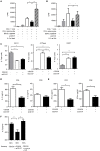
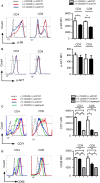
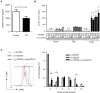
We show here that soluble CD137 (sCD137), the alternately spliced gene product of Tnfsfr9, effectively treats acute type 1 diabetes (T1D) in nonobese diabetic (NOD) mice. sCD137 significantly delayed development of end-stage disease, preserved insulin+ islet beta cells, and prevented progression to end-stage T1D in some mice. We demonstrate that sCD137 induces CD4+ T cell anergy, suppressing antigen-specific T cell proliferation and IL-2/IFN-γ secretion. Exogenous IL-2 reversed the sCD137 anergy effect. sCD137 greatly reduces inflammatory cytokine production by CD8 effector memory T cells, critical mediators of beta cell damage. We demonstrate that human T1D patients have decreased serum sCD137 compared to age-matched controls (as do NOD mice compared to NOD congenic mice expressing a protective Tnfsfr9 allele), that human sCD137 is secreted by regulatory T cells (Tregs; as in mice), and that human sCD137 induces T cell suppression in human T cells. These findings provide a rationale for further investigation of sCD137 as a treatment for T1D and other T cell-mediated autoimmune diseases.
Keywords: T cell anergy; T cells; autoimmunity; soluble CD137; type 1 diabetes.
Copyright © 2019 Itoh, Ortiz, Kachapati, Wu, Adams, Bednar, Mukherjee, Chougnet, Mittler, Chen, Dolan and Ridgway.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

