Unique Structural Features of Mule Deer Prion Protein Provide Insights into Chronic Wasting Disease
- PMID: 31788624
- PMCID: PMC6882122
- DOI: 10.1021/acsomega.9b02824
Unique Structural Features of Mule Deer Prion Protein Provide Insights into Chronic Wasting Disease
Abstract
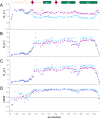
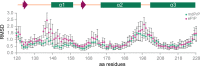
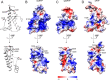
Chronic wasting disease (CWD) is a highly infectious prion disease of cervids. Accumulation of prions, the disease-specific structural conformers of the cellular prion protein (PrPC), in the central nervous system, is the key pathological event of the disorder. The analysis of cervid PrPC sequences revealed the existence of polymorphism at position 226, in which deer PrP contains glutamine (Q), whereas elk PrP contains glutamate (E). The effects of this polymorphism on CWD are still unknown. We determined the high-resolution nuclear magnetic resonance structure of the mule deer prion protein that was compared to previously published PrP structures of elk and white-tailed deer. We found that the polymorphism Q226E could influence the long-range intramolecular interactions and packing of the β2-α2 loop and the C-terminus of the α3 helix of cervid PrP structures. This solvent-accessible epitope is believed to be involved in prion conversion. Additional differences were observed at the beginning of the well-defined C-terminus domain, in the α2-α3 region, and in its interactions with the α1 helix. Here, we highlight the importance of the PrP structure in prion susceptibility and how single amino acid differences might influence the overall protein folding.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

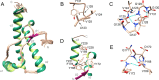

References
-
- Spraker T. R.; Miller M. W.; Williams E. S.; Getzy D. M.; Adrian W. J.; Schoonveld G. G.; Spowart R. A.; O’Rourke K. I.; Miller J. M.; Merz P. A. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J. Wildl. Dis. 1997, 33, 1–6. 10.7589/0090-3558-33.1.1. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

