Mitochondrial mass governs the extent of human T cell senescence
- PMID: 31788930
- PMCID: PMC6996952
- DOI: 10.1111/acel.13067
Mitochondrial mass governs the extent of human T cell senescence
Abstract
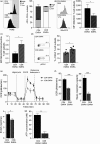
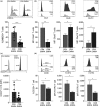
The susceptibility of human CD4+ and CD8+ T cells to senesce differs, with CD8+ T cells acquiring an immunosenescent phenotype faster than the CD4+ T cell compartment. We show here that it is the inherent difference in mitochondrial content that drives this phenotype, with senescent human CD4+ T cells displaying a higher mitochondrial mass. The loss of mitochondria in the senescent human CD8+ T cells has knock-on consequences for nutrient usage, metabolism and function. Senescent CD4+ T cells uptake more lipid and glucose than their CD8+ counterparts, leading to a greater metabolic versatility engaging either an oxidative or a glycolytic metabolism. The enhanced metabolic advantage of senescent CD4+ T cells allows for more proliferation and migration than observed in the senescent CD8+ subset. Mitochondrial dysfunction has been linked to both cellular senescence and aging; however, it is still unclear whether mitochondria play a causal role in senescence. Our data show that reducing mitochondrial function in human CD4+ T cells, through the addition of low-dose rotenone, causes the generation of a CD4+ T cell with a CD8+ -like phenotype. Therefore, we wish to propose that it is the inherent metabolic stability that governs the susceptibility to an immunosenescent phenotype.
Keywords: T cell; aging; metabolism; mitochondria; senescence.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures



References
-
- Appay, V. , van Lier, R. A. , Sallusto, F. , & Roederer, M. (2008). Phenotype and function of human T lymphocyte subsets: Consensus and issues. Cytometry A, 73, 975–983. - PubMed
-
- Brainard, D. M. , Tager, A. M. , Misdraji, J. , Frahm, N. , Lichterfeld, M. , Draenert, R. , … Luster, A. D. (2007). Decreased CXCR3+ CD8 T cells in advanced human immunodeficiency virus infection suggest that a homing defect contributes to cytotoxic T‐lymphocyte dysfunction. Journal of Virology, 81, 8439–8450. 10.1128/JVI.00199-07 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

