APP-derived peptides reflect neurodegeneration in frontotemporal dementia
- PMID: 31789459
- PMCID: PMC6917306
- DOI: 10.1002/acn3.50948
APP-derived peptides reflect neurodegeneration in frontotemporal dementia
Abstract
Objective: We aimed to investigate the relationship between cerebrospinal fluid levels (CSF) of amyloid precursor protein (APP)-derived peptides related to the amyloidogenic pathway, cortical thickness, neuropsychological performance, and cortical gene expression profiles in frontotemporal lobar degeneration (FTLD)-related syndromes, Alzheimer's disease (AD), and healthy controls.
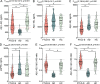
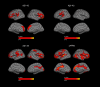
Methods: We included 214 participants with CSF available recruited at two centers: 93 with FTLD-related syndromes, 57 patients with AD, and 64 healthy controls. CSF levels of amyloid β (Aβ)1-42, Aβ1-40, Aβ1-38, and soluble β fragment of APP (sAPPβ) were centrally analyzed. We compared CSF levels of APP-derived peptides between groups and, we studied the correlation between CSF biomarkers, cortical thickness, and domain-specific cognitive composites in each group. Then, we explored the relationship between cortical thickness, CSF levels of APP-derived peptides, and regional gene expression profile using a brain-wide regional gene expression data in combination with gene set enrichment analysis.
Results: The CSF levels of Aβ1-40, Aβ1-38, and sAPPβ were lower in the FTLD-related syndromes group than in the AD and healthy controls group. CSF levels of all APP-derived peptides showed a positive correlation with cortical thickness and the executive cognitive composite in the FTLD-related syndromes group but not in the healthy control or AD groups. In the cortical regions where we observed a significant association between cortical thickness and CSF levels of APP-derived peptides, we found a reduced expression of genes related to synaptic function.
Interpretation: APP-derived peptides in CSF may reflect FTLD-related neurodegeneration. This observation has important implications as Aβ1-42 levels are considered an indirect biomarker of cerebral amyloidosis.
© 2019 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- SLT002/16/00408/Health Department of the Government of Catalonia/International
- PI11/02526/Instituto de Salud Carlos III/International
- PI14/01126/Instituto de Salud Carlos III/International
- PI17/01019/Instituto de Salud Carlos III/International
- PI13/01532/Instituto de Salud Carlos III/International
- PI16/01825/Instituto de Salud Carlos III/International
- PI15/01618/Instituto de Salud Carlos III/International
- PI18/00435/Instituto de Salud Carlos III/International
- PI14/1561/Instituto de Salud Carlos III/International
- PI17/01896/Instituto de Salud Carlos III/International
- AC14/00013/Instituto de Salud Carlos III/International
- CIBERNED Program/International
- Fondo Europeo de Desarrollo Regional/International
- Unión Europea/International
- Una manera de hacer Europa/International
- 20141210/Marató TV3/International
- 044412/Marató TV3/International
- 20143710/Marató TV3/International
- 20143810/Marató TV3/International
- 2014SGR-0235/Generalitat de Catalunya/International
- SLT006/17/125/Generalitat de Catalunya/International
- SLT006/17/00119/Generalitat de Catalunya/International
- BBVA Foundation/International
- Fundació Bancaria La Caixa to Rafael Blesa/International
- Acción estratégica en Salud 2013-2016/International
- European Social Fund/International
- Global Brain Health Institute/International
- SLT002/16/00329/Departament de Salut de la Generalitat de Catalunya/International
LinkOut - more resources
Full Text Sources
Medical

