Urea Derivatives in Modern Drug Discovery and Medicinal Chemistry
- PMID: 31789518
- PMCID: PMC7266097
- DOI: 10.1021/acs.jmedchem.9b01541
Urea Derivatives in Modern Drug Discovery and Medicinal Chemistry
Abstract
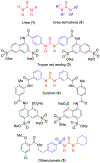
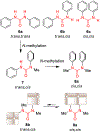
The urea functionality is inherent to numerous bioactive compounds, including a variety of clinically approved therapies. Urea containing compounds are increasingly used in medicinal chemistry and drug design in order to establish key drug-target interactions and fine-tune crucial drug-like properties. In this perspective, we highlight physicochemical and conformational properties of urea derivatives. We provide outlines of traditional reagents and chemical procedures for the preparation of ureas. Also, we discuss newly developed methodologies mainly aimed at overcoming safety issues associated with traditional synthesis. Finally, we provide a broad overview of urea-based medicinally relevant compounds, ranging from approved drugs to recent medicinal chemistry developments.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
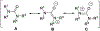
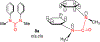
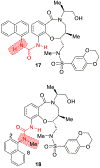
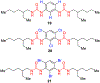
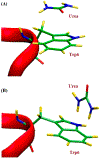
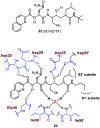
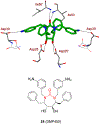
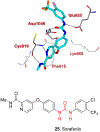
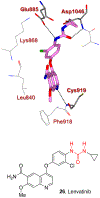
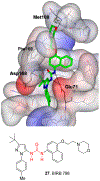
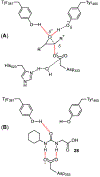
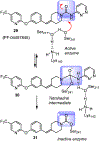
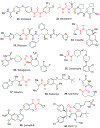
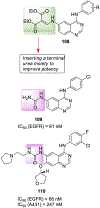
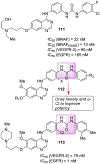
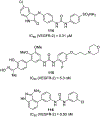
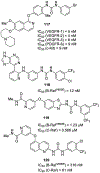
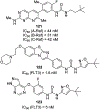
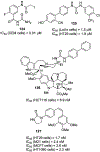
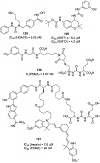
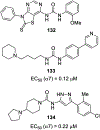
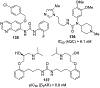

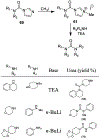
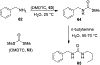
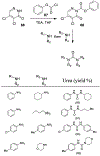
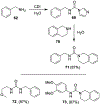
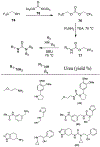
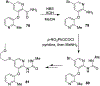
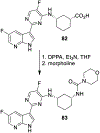
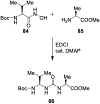
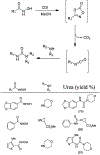
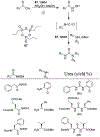
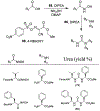
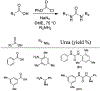
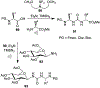
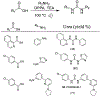
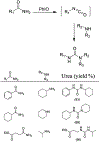
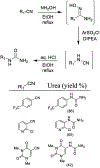
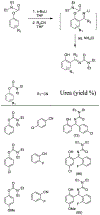
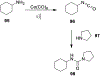
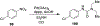
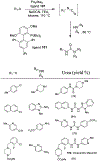
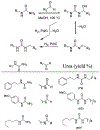
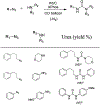
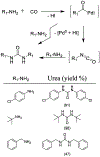
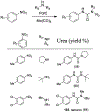
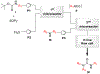
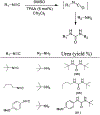
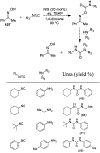
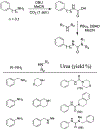
Similar articles
-
The Curtius Rearrangement: Applications in Modern Drug Discovery and Medicinal Chemistry.ChemMedChem. 2018 Nov 20;13(22):2351-2373. doi: 10.1002/cmdc.201800518. Epub 2018 Oct 11. ChemMedChem. 2018. PMID: 30187672 Free PMC article. Review.
-
Ureas: Applications in Drug Design.Curr Med Chem. 2017;24(6):622-651. doi: 10.2174/0929867323666161129124915. Curr Med Chem. 2017. PMID: 27897114 Review.
-
The Medicinal Chemistry in the Era of Machines and Automation: Recent Advances in Continuous Flow Technology.J Med Chem. 2020 Jul 9;63(13):6624-6647. doi: 10.1021/acs.jmedchem.9b01956. Epub 2020 Feb 21. J Med Chem. 2020. PMID: 32049517 Free PMC article. Review.
-
Recent applications of click chemistry in drug discovery.Expert Opin Drug Discov. 2019 Aug;14(8):779-789. doi: 10.1080/17460441.2019.1614910. Epub 2019 May 16. Expert Opin Drug Discov. 2019. PMID: 31094231 Review.
-
Recent developments in the discovery of protein kinase inhibitors from the urea class.Curr Opin Drug Discov Devel. 2004 Sep;7(5):600-16. Curr Opin Drug Discov Devel. 2004. PMID: 15503863 Review.
Cited by
-
Late-Stage Carbon-14 Labeling and Isotope Exchange: Emerging Opportunities and Future Challenges.JACS Au. 2022 Jun 7;2(6):1234-1251. doi: 10.1021/jacsau.2c00030. eCollection 2022 Jun 27. JACS Au. 2022. PMID: 35783167 Free PMC article. Review.
-
Synthesis and Selective Functionalization of Thiadiazine 1,1-Dioxides with Efficacy in a Model of Huntington's Disease.ACS Med Chem Lett. 2020 Feb 20;11(5):984-990. doi: 10.1021/acsmedchemlett.0c00018. eCollection 2020 May 14. ACS Med Chem Lett. 2020. PMID: 32435415 Free PMC article.
-
Discovery of Novel Pyridin-2-yl Urea Inhibitors Targeting ASK1 Kinase and Its Binding Mode by Absolute Protein-Ligand Binding Free Energy Calculations.Int J Mol Sci. 2025 Feb 12;26(4):1527. doi: 10.3390/ijms26041527. Int J Mol Sci. 2025. PMID: 40003993 Free PMC article.
-
CD, UV, and In Silico Insights on the Effect of 1,3-Bis(1'-uracilyl)-2-propanone on Serum Albumin Structure.Biomolecules. 2022 Aug 3;12(8):1071. doi: 10.3390/biom12081071. Biomolecules. 2022. PMID: 36008965 Free PMC article.
-
Isocyanate-based multicomponent reactions.RSC Adv. 2024 Dec 12;14(53):39253-39267. doi: 10.1039/d4ra04152f. eCollection 2024 Dec 10. RSC Adv. 2024. PMID: 39670166 Free PMC article. Review.
References
-
- Wöhler F Ueber künstliche bildung des harnstoffs. Ann. Phys 1828, 88, 253–256.
-
- Ramberg PJ The death of vitalism and the birth of organic chemistry: Wohler’s urea synthesis and the disciplinary identity of organic chemistry. Ambix 2000, 47, 170–195. - PubMed
-
- Burger A History and Economics of Medicinal Chemistry In Medicinal Chemistry, 3rd ed.; Burger A, Ed.; John Wiley & Sons: Hoboken, NJ, 1970; Vol 1, pp 4–19.
-
- Lombardino JG; Lowe JA 3rd The role of the medicinal chemist in drug discovery-then and now. Nat. Rev. Drug Discovery 2004, 3, 853–862. - PubMed
-
- Gallou I Unsymmetrical ureas. Synthetic methodologies and application in drug design. Org. Prep. Proced. Int 2007, 39, 355–383.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

