Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis
- PMID: 31789672
- PMCID: PMC7265982
- DOI: 10.1097/QCO.0000000000000620
Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis
Abstract
Purpose of review: The cause of bacterial vaginosis, the most common cause of vaginal discharge in women, remains controversial. We recently published an updated conceptual model on bacterial vaginosis pathogenesis, focusing on the roles of Gardnerella vaginalis and Prevotella bivia as early colonizers and Atopobium vaginae and other bacterial vaginosis-associated bacteria (BVAB) as secondary colonizers in this infection. In this article, we extend the description of our model to include a discussion on the role of host-vaginal microbiota interactions in bacterial vaginosis pathogenesis.
Recent findings: Although G. vaginalis and P. bivia are highly abundant in women with bacterial vaginosis, neither induce a robust inflammatory response from vaginal epithelial cells. These early colonizers may be evading the immune system while establishing the bacterial vaginosis biofilm. Secondary colonizers, including A. vaginae, Sneathia spp., and potentially other BVAB are more potent stimulators of the host-immune response to bacterial vaginosis and likely contribute to its signs and symptoms as well as its adverse outcomes.
Summary: Elucidating the cause of bacterial vaginosis has important implications for diagnosis and treatment. Our current bacterial vaginosis pathogenesis model provides a framework for key elements that should be considered when designing and testing novel bacterial vaginosis diagnostics and therapeutics.
Figures
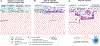
References
-
- Swidsinski A, Mendling W, Loening-Baucke V, et al. Adherent biofilms in bacterial vaginosis. Obstet Gynecol 2005;106(5 Pt 1):1013–23. - PubMed
-
-
Peebles K, Velloza J, Balkus JE, et al. High Global Burden and Costs of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Sex Transm Dis 2019;46(5):304–11.
* This article details the global prevalence of BV and estimates the direct medical costs of treating symptomatic BV.
-
-
- Hillier SL, Marrazzo J, Holmes KK. Bacterial vaginosis In: Holmes KK, Sparling PF, Mardh PA, et al., editors. Sexually transmitted diseases. 4th edition. New York: McGraw-Hill; 2008. p. 737–68.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

